A tactical nanomissile mobilizing antitumor immunity enables neoadjuvant chemo-immunotherapy to minimize postsurgical tumor metastasis and recurrence
- PMID: 36873172
- PMCID: PMC9979264
- DOI: 10.1016/j.apsb.2022.09.017
A tactical nanomissile mobilizing antitumor immunity enables neoadjuvant chemo-immunotherapy to minimize postsurgical tumor metastasis and recurrence
Abstract
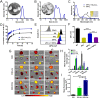
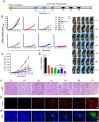
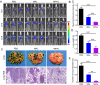
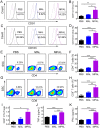
Neoadjuvant chemotherapy has become an indispensable weapon against high-risk resectable cancers, which benefits from tumor downstaging. However, the utility of chemotherapeutics alone as a neoadjuvant agent is incapable of generating durable therapeutic benefits to prevent postsurgical tumor metastasis and recurrence. Herein, a tactical nanomissile (TALE), equipped with a guidance system (PD-L1 monoclonal antibody), ammunition (mitoxantrone, Mit), and projectile bodies (tertiary amines modified azobenzene derivatives), is designed as a neoadjuvant chemo-immunotherapy setting, which aims at targeting tumor cells, and fast-releasing Mit owing to the intracellular azoreductase, thereby inducing immunogenic tumor cells death, and forming an in situ tumor vaccine containing damage-associated molecular patterns and multiple tumor antigen epitopes to mobilize the immune system. The formed in situ tumor vaccine can recruit and activate antigen-presenting cells, and ultimately increase the infiltration of CD8+ T cells while reversing the immunosuppression microenvironment. Moreover, this approach provokes a robust systemic immune response and immunological memory, as evidenced by preventing 83.3% of mice from postsurgical metastasis or recurrence in the B16-F10 tumor mouse model. Collectively, our results highlight the potential of TALE as a neoadjuvant chemo-immunotherapy paradigm that can not only debulk tumors but generate a long-term immunosurveillance to maximize the durable benefits of neoadjuvant chemotherapy.
Keywords: Azobenzene derivatives; Chemotherapy; Immunotherapy; Melanoma; Mitoxantrone; Neoadjuvant; Reduction responsive; Vaccine.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors report no conflicts of interest.
Figures




References
-
- Montemurro F., Nuzzolese I., Ponzone R. Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expet Opin Pharmacother. 2020;21:1071–1082. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

