Carboxylesterase 1 family knockout alters drug disposition and lipid metabolism
- PMID: 36873183
- PMCID: PMC9978993
- DOI: 10.1016/j.apsb.2022.10.017
Carboxylesterase 1 family knockout alters drug disposition and lipid metabolism
Abstract
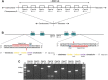
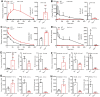
The mammalian carboxylesterase 1 (Ces1/CES1) family comprises several enzymes that hydrolyze many xenobiotic chemicals and endogenous lipids. To investigate the pharmacological and physiological roles of Ces1/CES1, we generated Ces1 cluster knockout (Ces1 -/- ) mice, and a hepatic human CES1 transgenic model in the Ces1 -/- background (TgCES1). Ces1 -/- mice displayed profoundly decreased conversion of the anticancer prodrug irinotecan to SN-38 in plasma and tissues. TgCES1 mice exhibited enhanced metabolism of irinotecan to SN-38 in liver and kidney. Ces1 and hCES1 activity increased irinotecan toxicity, likely by enhancing the formation of pharmacodynamically active SN-38. Ces1 -/- mice also showed markedly increased capecitabine plasma exposure, which was moderately decreased in TgCES1 mice. Ces1 -/- mice were overweight with increased adipose tissue, white adipose tissue inflammation (in males), a higher lipid load in brown adipose tissue, and impaired blood glucose tolerance (in males). These phenotypes were mostly reversed in TgCES1 mice. TgCES1 mice displayed increased triglyceride secretion from liver to plasma, together with higher triglyceride levels in the male liver. These results indicate that the carboxylesterase 1 family plays essential roles in drug and lipid metabolism and detoxification. Ces1 -/- and TgCES1 mice will provide excellent tools for further study of the in vivo functions of Ces1/CES1 enzymes.
Keywords: Adipose tissue; Capecitabine; Ces1/CES1 enzymes; Inflammation; Irinotecan; Lipid homeostasis; Mouse models; Triglyceride mobilization.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures









Similar articles
-
Natural deletion of mouse carboxylesterases Ces1c/d/e impacts drug metabolism and metabolic syndrome development.Biomed Pharmacother. 2023 Aug;164:114956. doi: 10.1016/j.biopha.2023.114956. Epub 2023 May 31. Biomed Pharmacother. 2023. PMID: 37267638
-
OATP1A/1B transporters affect irinotecan and SN-38 pharmacokinetics and carboxylesterase expression in knockout and humanized transgenic mice.Mol Cancer Ther. 2014 Feb;13(2):492-503. doi: 10.1158/1535-7163.MCT-13-0541. Epub 2013 Nov 5. Mol Cancer Ther. 2014. PMID: 24194565
-
Association of carboxylesterase 1A genotypes with irinotecan pharmacokinetics in Japanese cancer patients.Br J Clin Pharmacol. 2010 Aug;70(2):222-33. doi: 10.1111/j.1365-2125.2010.03695.x. Br J Clin Pharmacol. 2010. PMID: 20653675 Free PMC article.
-
The role of adipose triglyceride lipase in lipid and glucose homeostasis: lessons from transgenic mice.Lipids Health Dis. 2019 Nov 22;18(1):204. doi: 10.1186/s12944-019-1151-z. Lipids Health Dis. 2019. PMID: 31757217 Free PMC article. Review.
-
Potential Pharmacokinetic Herb-Drug Interactions: Have we Overlooked the Importance of Human Carboxylesterases 1 and 2?Curr Drug Metab. 2019;20(2):130-137. doi: 10.2174/1389200219666180330124050. Curr Drug Metab. 2019. PMID: 29600756 Review.
Cited by
-
Intestinal human carboxylesterase 2 (CES2) expression rescues drug metabolism and most metabolic syndrome phenotypes in global Ces2 cluster knockout mice.Acta Pharmacol Sin. 2025 Mar;46(3):777-793. doi: 10.1038/s41401-024-01407-4. Epub 2024 Nov 4. Acta Pharmacol Sin. 2025. PMID: 39496863 Free PMC article.
-
Carboxylesterase 1 regulates peroxisome proliferator-activated receptor gamma to inhibit the growth and metastasis of breast cancer cells.J Mol Histol. 2025 May 26;56(3):167. doi: 10.1007/s10735-025-10446-y. J Mol Histol. 2025. PMID: 40418235
-
Targeted inhibition of Gus-expressing Enterococcus faecalis to promote intestinal stem cell and epithelial renovation contributes to the relief of irinotecan chemotoxicity by dehydrodiisoeugenol.Acta Pharm Sin B. 2024 Dec;14(12):5286-5304. doi: 10.1016/j.apsb.2024.09.018. Epub 2024 Oct 18. Acta Pharm Sin B. 2024. PMID: 39807321 Free PMC article.
-
Hypercholesterolemia and the role of lipid metabolism gene CES1 in immune infiltration promote central nervous system relapse in acute myeloid leukemia.Front Immunol. 2025 Jul 23;16:1575472. doi: 10.3389/fimmu.2025.1575472. eCollection 2025. Front Immunol. 2025. PMID: 40771823 Free PMC article.
-
Melatonin: a natural guardian in cancer treatment.Front Pharmacol. 2025 Jul 18;16:1617508. doi: 10.3389/fphar.2025.1617508. eCollection 2025. Front Pharmacol. 2025. PMID: 40756978 Free PMC article. Review.
References
-
- Jin Q., Song L.L., Ding L.L., Zhang J., Wang D.D., Song Y.Q., et al. High-throughput optical assays for sensing serine hydrolases in living systems and their applications. TrAC, Trends Anal Chem. 2022
LinkOut - more resources
Full Text Sources
Miscellaneous

