Acetyl-L-carnitine for the prevention of taxane-induced neuropathy in patients with breast cancer: a systematic review and meta-analysis
- PMID: 36873277
- PMCID: PMC9976057
- DOI: 10.4103/1735-5362.367791
Acetyl-L-carnitine for the prevention of taxane-induced neuropathy in patients with breast cancer: a systematic review and meta-analysis
Abstract
Background and purpose: Peripheral neuropathy is one of the most prevalent and undesirable side effects of taxane-containing chemotherapy regimens. This study aimed to investigate the effect of acetyl-L-carnitine (ALC) on the prevention of taxane-induced neuropathy (TIN).
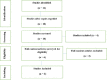
Experimental approach: MEDLINE, PubMed, Cochrane Library, Embase, Web of Science, and Google scholar were systemically applied as electronic databases from 2010 to 2019. The current systematic review was carried out based on the main considerations of PRISMA preferential reporting items for systematic review and meta-analyses. Since there was no significant discrepancy, the random-effect model was used for 12-24 weeks' analysis (I2 = 0%, P = 0.999).
Findings/results: Twelve related titles and abstracts were found during the search, 6 of them were excluded in the first phase. In the second phase, the full text of the remaining 6 articles was comprehensively evaluated and 3 papers were rejected. Finally, 3 articles complied with the inclusion criteria and pooled analyses. The meta-analysis showed a risk ratio of 0.796 (95% CI between 0.486 and 1.303), so, the effects model was used for 12-24 weeks' analysis (I2 = 0%, P = 0.999) since no significant discrepancies were observed. There was no evidence of ALC's positive effect on the prevention of TIN during 12 weeks, and it was revealed that ALC significantly increased TIN in 24 weeks.
Conclusion and implications: According to our findings, the hypothesis that ALC had a positive effect on preventing TIN in 12 weeks has not been proved; however, ALC led to an increase in the TIN in 24 weeks.
Keywords: Acetyl-L-carnitine; Breast cancer; Chemotherapy; Neuropathy; Taxane.
Copyright: © 2023 Research in Pharmaceutical Sciences.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Figures

References
-
- Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–983. doi: 10.1200/JCO.2003.02.063. - DOI - PubMed
-
- Zhi WI, Chen P, Kwon A, Chen C, Harte SE, Piulson L, et al. Chemotherapy-induced peripheral neuropathy (CIPN) in breast cancer survivors: a comparison of patient-reported outcomes and quantitative sensory testing. Breast Cancer Res Treat. 2019;178(3):587–595. doi: 10.1007/s10549-019-05416-4. - DOI - PMC - PubMed
-
- Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125(3):767–774. doi: 10.1007/s10549-010-1278-0. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
