Vestibular assessment in sudden sensorineural hearing loss: Role in the prediction of hearing outcome and in the early detection of vascular and hydropic pathomechanisms
- PMID: 36873440
- PMCID: PMC9975513
- DOI: 10.3389/fneur.2023.1127008
Vestibular assessment in sudden sensorineural hearing loss: Role in the prediction of hearing outcome and in the early detection of vascular and hydropic pathomechanisms
Abstract
Introduction: Predicting hearing outcome in sudden sensorineural hearing loss (SSNHL) is challenging, as well as detecting the underlying pathomechanisms. SSNHL could be associated with vestibular damage since cochleo-vestibular structures share the same vascularization, along with being in close anatomical proximity. Whereas viral inflammations and autoimmune/vascular disorders most likely represent the involved aetiologies, early-stage Menière's disease (MD) can also present with SSNHL. Since an early treatment could beneficially influence hearing outcome, understanding the possible etiology plays a pivotal role in orienting the most appropriate treatment. We aimed to evaluate the extent of vestibular damage in patients presenting with SSNHL with or without vertigo, investigate the prognostic role of vestibular dysfunctions on hearing recovery and detect specific lesion patterns related to the underlying pathomechanisms.
Methods: We prospectively evaluated 86 patients with SSNHL. Audio-vestibular investigation included pure-tone/speech/impedance audiometry, cervical/ocular-VEMPs, vHIT and video-Frenzel examination. White matter lesions (WML) were evaluated on brain-MRI. Patients were followed-up and divided into "SSNHL-no-vertigo," "SSNHL+vertigo" and "MD" subgroups.
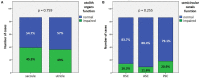
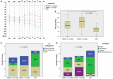
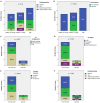
Results: Hearing was more impaired in "SSNHL+vertigo" patients who exhibited either down-sloping or flat-type audiograms, and was less impaired in "MD" where low frequencies were mostly impaired (p < 0.001). Otolith receptors were more frequently involved than semicircular canals (SCs). Although the "SSNHL-no-vertigo" subgroup exhibited the lowest vestibular impairment (p < 0.001), 52% of patients developed otolith dysfunctions and 72% developed nystagmus. Only "MD" subjects showed anterior SC impairment and upbeating spontaneous/positional nystagmus. They more frequently exhibited cervical-VEMPs frequency tuning (p = 0.036) and ipsilesional spontaneous nystagmus (p < 0.001). "SSNHL+vertigo" subjects presented with more frequently impaired cervical-VEMPs and posterior SC and with higher number of impaired receptors (p < 0.001). They mainly exhibited contralesional spontaneous and vibration-induced nystagmus (p < 0.05) and only they showed the highest WML score and "vascular" lesion patterns (p < 0.001). Concerning the outcomes, hearing was better in "MD" and worse in "SSNHL+vertigo" (p < 0.001). Hearing recovery was mostly affected by cervical-VEMPs impairment and the number of involved receptors (p < 0.05). Patients with "vascular" lesion patterns presented with the highest HL degree and WML score (p ≤ 0.001), while none of them exhibited a complete hearing recovery (p = 0.026).
Conclusions: Our data suggest that vestibular evaluation in SSNHL can provide useful information on hearing recovery and underlying aetiologies.
Keywords: Menière's disease; labyrinthine ischemia; spontaneous nystagmus; sudden sensorineunal hearing loss; vestibular evoked myogenic potentials (VEMPs); vestibulo-ocular reflex (VOR); video head impulse (vHIT).
Copyright © 2023 Castellucci, Botti, Delmonte, Bettini, Lusetti, Brizzi, Ruberto, Gamberini, Martellucci, Malara, Armato, Renna, Ghidini and Bianchin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
LinkOut - more resources
Full Text Sources

