Chemical Basis of Combination Therapy to Combat Antibiotic Resistance
- PMID: 36873689
- PMCID: PMC9975838
- DOI: 10.1021/jacsau.2c00532
Chemical Basis of Combination Therapy to Combat Antibiotic Resistance
Abstract
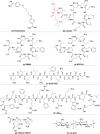
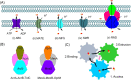
The antimicrobial resistance crisis is a global health issue requiring discovery and development of novel therapeutics. However, conventional screening of natural products or synthetic chemical libraries is uncertain. Combination therapy using approved antibiotics with inhibitors targeting innate resistance mechanisms provides an alternative strategy to develop potent therapeutics. This review discusses the chemical structures of effective β-lactamase inhibitors, outer membrane permeabilizers, and efflux pump inhibitors that act as adjuvant molecules of classical antibiotics. Rational design of the chemical structures of adjuvants will provide methods to impart or restore efficacy to classical antibiotics for inherently antibiotic-resistant bacteria. As many bacteria have multiple resistance pathways, adjuvant molecules simultaneously targeting multiple pathways are promising approaches to combat multidrug-resistant bacterial infections.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
