Enantioselective construction of triaryl-substituted all-carbon quaternary stereocenters via organocatalytic arylation of oxindoles with azonaphthalenes
- PMID: 36873834
- PMCID: PMC9977417
- DOI: 10.1039/d2sc07103g
Enantioselective construction of triaryl-substituted all-carbon quaternary stereocenters via organocatalytic arylation of oxindoles with azonaphthalenes
Abstract
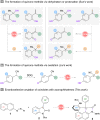
Azonaphthalenes have been verified as a class of effective arylation reagents in a variety of asymmetric transformations. Here a highly efficient approach to construct triaryl-substituted all-carbon quaternary stereocenters through chiral phosphoric acid-catalyzed enantioselective arylation of 3-aryl-2-oxindoles with azonaphthalenes is disclosed. This chemistry is scalable and displays excellent functional group tolerance, furnishing a series of 3,3-disubstituted 2-oxindole derivatives in good yields with excellent enantiocontrol. Preliminary mechanistic data suggest that the initially formed direct addition intermediate undergoes intramolecular annulation under acidic reaction conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Liu Y. Han S.-J. Liu W.-B. Stoltz B. M. Acc. Chem. Res. 2015;48:740–751. doi: 10.1021/ar5004658. - DOI - PMC - PubMed
- Han S.-J. Vogt F. Krishnan S. May J. A. Gatti M. Virgil S. C. Stoltz B. M. Org. Lett. 2014;16:3316–3319. doi: 10.1021/ol5013263. - DOI - PMC - PubMed
- Han S.-J. Vogt F. May J. A. Krishnan S. Gatti M. Virgil S. C. Stoltz B. M. J. Org. Chem. 2015;80:528–547. doi: 10.1021/jo502534g. - DOI - PMC - PubMed
-
- Uddin M. K. Reignier S. G. Coulter T. Montalbetti C. Grånäs C. Butcher S. Krog-Jensen C. Felding J. Bioorg. Med. Chem. Lett. 2007;17:2854–2857. doi: 10.1016/j.bmcl.2007.02.060. - DOI - PubMed
- Paira P. Hazra A. Kumar S. Paira R. Sahu K. B. Naskar S. saha P. Mondal S. Maity A. Banerjee S. Mondal N. B. Bioorg. Med. Chem. Lett. 2009;19:4786–4789. doi: 10.1016/j.bmcl.2009.06.049. - DOI - PubMed
-
- Corey E. J. Guzman-Perez A. Angew. Chem., Int. Ed. 1998;37:388–401. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V. - DOI - PubMed
- Douglas C. J. Overman L. E. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5363–5367. doi: 10.1073/pnas.0307113101. - DOI - PMC - PubMed
- Trost B. M. Jiang C. Synthesis. 2006;3:369–396. doi: 10.1055/s-2006-926302. - DOI
- Hawner C. Alexakis A. Chem. Commun. 2010;46:7295–7306. doi: 10.1039/C0CC02309D. - DOI - PubMed
- Das J. P. Marek I. Chem. Commun. 2011;47:4593–4623. doi: 10.1039/C0CC05222A. - DOI - PubMed
-
- Evans P. A. Oliver S. Chae J. J. Am. Chem. Soc. 2012;134:19314–19317. doi: 10.1021/ja306602g. - DOI - PubMed
- Gao L. Kang B. C. Ryu D. H. J. Am. Chem. Soc. 2013;135:14556–14559. doi: 10.1021/ja408196g. - DOI - PubMed
- Quasdorf K. W. Overman L. E. Nature. 2014;516:181–191. doi: 10.1038/nature14007. - DOI - PMC - PubMed
- Marek I. Minko Y. Pasco M. Mejuch T. Gilboa N. Chechik H. Das J. P. J. Am. Chem. Soc. 2014;136:2682–2694. doi: 10.1021/ja410424g. - DOI - PubMed
- Hojoh K. Shido Y. Ohmiya H. Sawamura M. Angew. Chem., Int. Ed. 2014;53:4954–4958. doi: 10.1002/anie.201402386. - DOI - PubMed
- Lin J.-S. Li T.-T. Liu J.-R. Jiao G.-Y. Gu Q.-S. Cheng J.-T. Guo Y.-L. Hong X. Liu X.-Y. J. Am. Chem. Soc. 2019;141:1074–1083. doi: 10.1021/jacs.8b11736. - DOI - PubMed
- Ma D. Miao C.-B. Sun J. J. Am. Chem. Soc. 2019;141:13783–13787. doi: 10.1021/jacs.9b07514. - DOI - PubMed
-
- Zhao W. Wang Z. Chu B. Sun J. Angew. Chem., Int. Ed. 2015;54:1910–1913. doi: 10.1002/anie.201405252. - DOI - PubMed
- Wang Z. Wong Y. F. Sun J. Angew. Chem., Int. Ed. 2015;54:13711–13714. doi: 10.1002/anie.201506701. - DOI - PubMed
- Wang Z. Ai F. Wang Z. Zhao W. Zhu G. Lin Z. Sun J. J. Am. Chem. Soc. 2015;137:383–389. doi: 10.1021/ja510980d. - DOI - PubMed
- Li Z. Li Y. Li X. Wu M. He M.-L. Sun J. Chem. Sci. 2021;12:11793–11798. doi: 10.1039/D1SC03324G. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

