Pathogenic gene variants in CCDC39, CCDC40, RSPH1, RSPH9, HYDIN, and SPEF2 cause defects of sperm flagella composition and male infertility
- PMID: 36873931
- PMCID: PMC9981940
- DOI: 10.3389/fgene.2023.1117821
Pathogenic gene variants in CCDC39, CCDC40, RSPH1, RSPH9, HYDIN, and SPEF2 cause defects of sperm flagella composition and male infertility
Abstract
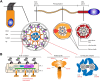
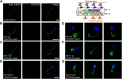
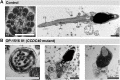
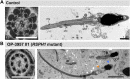
Primary Ciliary Dyskinesia (PCD) is a rare genetic disorder affecting the function of motile cilia in several organ systems. In PCD, male infertility is caused by defective sperm flagella composition or deficient motile cilia function in the efferent ducts of the male reproductive system. Different PCD-associated genes encoding axonemal components involved in the regulation of ciliary and flagellar beating are also reported to cause infertility due to multiple morphological abnormalities of the sperm flagella (MMAF). Here, we performed genetic testing by next generation sequencing techniques, PCD diagnostics including immunofluorescence-, transmission electron-, and high-speed video microscopy on sperm flagella and andrological work up including semen analyses. We identified ten infertile male individuals with pathogenic variants in CCDC39 (one) and CCDC40 (two) encoding ruler proteins, RSPH1 (two) and RSPH9 (one) encoding radial spoke head proteins, and HYDIN (two) and SPEF2 (two) encoding CP-associated proteins, respectively. We demonstrate for the first time that pathogenic variants in RSPH1 and RSPH9 cause male infertility due to sperm cell dysmotility and abnormal flagellar RSPH1 and RSPH9 composition. We also provide novel evidence for MMAF in HYDIN- and RSPH1-mutant individuals. We show absence or severe reduction of CCDC39 and SPEF2 in sperm flagella of CCDC39- and CCDC40-mutant individuals and HYDIN- and SPEF2-mutant individuals, respectively. Thereby, we reveal interactions between CCDC39 and CCDC40 as well as HYDIN and SPEF2 in sperm flagella. Our findings demonstrate that immunofluorescence microscopy in sperm cells is a valuable tool to identify flagellar defects related to the axonemal ruler, radial spoke head and the central pair apparatus, thus aiding the diagnosis of male infertility. This is of particular importance to classify the pathogenicity of genetic defects, especially in cases of missense variants of unknown significance, or to interpret HYDIN variants that are confounded by the presence of the almost identical pseudogene HYDIN2.
Keywords: CP-complex; MMAF; PCD; RS head; asthenozoospermia; axonemal ruler.
Copyright © 2023 Aprea, Wilken, Krallmann, Nöthe-Menchen, Olbrich, Loges, Dougherty, Bracht, Brenker, Kliesch, Strünker, Tüttelmann, Raidt and Omran.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
SPEF2- and HYDIN-Mutant Cilia Lack the Central Pair-associated Protein SPEF2, Aiding Primary Ciliary Dyskinesia Diagnostics.Am J Respir Cell Mol Biol. 2020 Mar;62(3):382-396. doi: 10.1165/rcmb.2019-0086OC. Am J Respir Cell Mol Biol. 2020. PMID: 31545650
-
A novel CCDC39 mutation causes multiple morphological abnormalities of the flagella in a primary ciliary dyskinesia patient.Reprod Biomed Online. 2021 Nov;43(5):920-930. doi: 10.1016/j.rbmo.2021.07.005. Epub 2021 Jul 22. Reprod Biomed Online. 2021. PMID: 34674941
-
Immunofluorescence Analysis and Diagnosis of Primary Ciliary Dyskinesia with Radial Spoke Defects.Am J Respir Cell Mol Biol. 2015 Oct;53(4):563-73. doi: 10.1165/rcmb.2014-0483OC. Am J Respir Cell Mol Biol. 2015. PMID: 25789548 Free PMC article.
-
Primary Ciliary Dyskinesia.2007 Jan 24 [updated 2025 May 22]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2007 Jan 24 [updated 2025 May 22]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301301 Free Books & Documents. Review.
-
Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: Genetic defects with normal and non-diagnostic ciliary ultrastructure.Ultrastruct Pathol. 2017 Nov-Dec;41(6):373-385. doi: 10.1080/01913123.2017.1362088. Epub 2017 Sep 15. Ultrastruct Pathol. 2017. PMID: 28915070 Free PMC article. Review.
Cited by
-
PCM1 orchestrates centrosomal and flagellar protein transport to promote sperm maturation.Commun Biol. 2025 Jun 7;8(1):885. doi: 10.1038/s42003-025-08319-x. Commun Biol. 2025. PMID: 40481240 Free PMC article.
-
Genetic etiological spectrum of sperm morphological abnormalities.J Assist Reprod Genet. 2024 Nov;41(11):2877-2929. doi: 10.1007/s10815-024-03274-8. Epub 2024 Oct 17. J Assist Reprod Genet. 2024. PMID: 39417902 Review.
-
Genetic Causes of Qualitative Sperm Defects: A Narrative Review of Clinical Evidence.Genes (Basel). 2024 May 8;15(5):600. doi: 10.3390/genes15050600. Genes (Basel). 2024. PMID: 38790229 Free PMC article. Review.
-
IQUB mutation induces radial spoke 1 deficiency causing asthenozoospermia with normal sperm morphology in humans and mice.Cell Commun Signal. 2025 Jan 23;23(1):41. doi: 10.1186/s12964-025-02043-z. Cell Commun Signal. 2025. PMID: 39849482 Free PMC article.
-
Full-length transcriptome analysis of male and female gonads in Japanese Eel (Anguilla japonica).BMC Genomics. 2025 Jan 30;26(1):89. doi: 10.1186/s12864-025-11279-5. BMC Genomics. 2025. PMID: 39885385 Free PMC article.
References
-
- American Thoracic Society European Respiratory Society (2005). ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 171, 912–930. 10.1164/rccm.200406-710ST - DOI - PubMed
-
- Antony D., Becker-Heck A., Zariwala M. A., Schmidts M., Onoufriadis A., Forouhan M., et al. (2013). Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum. Mutat. 34, 462–472. 10.1002/humu.22261 - DOI - PMC - PubMed
-
- Aprea I., Raidt J., Höben I. M., Loges N. T., Nöthe-Menchen T., Pennekamp P., et al. (2021b). Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility. PLoS Genet. 17, e1009306. 10.1371/journal.pgen.1009306 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

