Highly ordered CaO from cuttlefish bone calcination for the efficient adsorption of methylene blue from water
- PMID: 36874067
- PMCID: PMC9977829
- DOI: 10.3389/fchem.2023.1132464
Highly ordered CaO from cuttlefish bone calcination for the efficient adsorption of methylene blue from water
Abstract
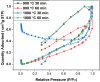
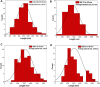
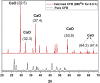
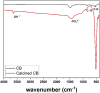
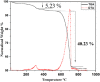
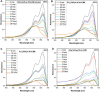
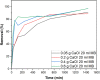
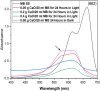
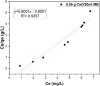
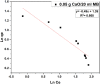
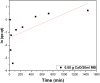
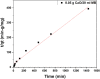
The aim of this study is to synthesize cheap and highly ordered CaO from cuttlefish bone (CFB) as a green alternative to conventional adsorbents such as activated carbon. This study focuses on the synthesis of highly ordered CaO via calcination of CFB, at two different temperatures (900 and 1000°C) and two holding times (0.5 and 1 h), as a potential green route for water remediation. The as-prepared highly ordered CaO was tested as an adsorbent using methylene blue (MB) as a model compound for dye contaminants in water. Different CaO adsorbent doses (0.05, 0.2, 0.4, and 0.6 g) were used, keeping the MB concentration fixed at 10 mg/L. The morphology and crystalline structure of the CFB before and after calcination was characterized via scanning electron microscope (SEM) and X-ray diffraction (XRD) analyses, while the thermal behavior and surface functionalities were characterized by thermogravimetric analysis (TGA) and Fourier transform infrared (FTIR) spectroscopy, respectively. Adsorption experiments using different doses of CaO synthesized at 900°C for 0.5 h showed an MB removal efficiency as high as 98% by weight using 0.4 g (adsorbent)/L(solution). Two different adsorption models, the Langmuir adsorption model and the Freundlich adsorption model, along with pseudo-first-order and pseudo-second-order kinetic models, were studied to correlate the adsorption data. The removal of MB via highly ordered CaO adsorption was better modeled by the Langmuir adsorption isotherm giving (R2 =0.93), thus proving a monolayer adsorption mechanism following pseudo-second-order kinetics (R2= 0.98), confirming that chemisorption reaction occurs between the MB dye molecule and CaO.
Keywords: calcination; calcium oxide (CaO); cuttlefish bone; methylene blue (dye); water remediation.
Copyright © 2023 Tagar, Volpe, Messineo and Volpe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Acosta P. I., Campedelli R. R., Correa E. L., Bazani H. A. G., Nishida E. N., Souza B. S., et al. (2020). Efficient production of biodiesel by using a highly active calcium oxide prepared in presence of pectin as heterogeneous catalyst. Fuel 271, 117651. 10.1016/j.fuel.2020.117651 - DOI
-
- Ahmed S., Mada V. V., Kamath V., Jeppu G. P. (2017). Characterization of activated carbon prepared from coconut shell using various reagents for A low cost water- filter. Int. J. Eng. Technol. 9, 180–188. 10.21817/ijet/2017/v9i3/170903s029 - DOI
-
- Amel K., Hassen M. A., Kerroum D. (2012). Isotherm and kinetics study of biosorption of cationic dye onto banana peel. Energy Procedia 19, 286–295. 10.1016/j.egypro.2012.05.208 - DOI
-
- Anand K. V., Reshma M., Kannan M., Selvan S. M., Chaturvedi S., Shalan A. E., et al. (2021). Preparation and characterization of calcium oxide nanoparticles from marine molluscan shell waste as nutrient source for plant growth. J. Nanostructure Chem. 11, 409–422. 10.1007/s40097-020-00376-4 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

