The effects of embryo splitting on Cdx2, Sox2, Oct4, and Nanog gene expression in mouse blastocysts
- PMID: 36874188
- PMCID: PMC9984146
- DOI: 10.22099/IJVR.2022.42487.6172
The effects of embryo splitting on Cdx2, Sox2, Oct4, and Nanog gene expression in mouse blastocysts
Abstract
Background: Embryo splitting is utilized in reproduction biotechnology. The blastomeres resulting from the splitting of an embryo in two-, four- or eight-cell stages can develop into separate embryos that are genetically similar to the other blastomeres.
Aims: The present work studied the effects of splitting on embryo pluripotent gene expression (Cdx2, Sox2, Oct4, and Nanog) in mice.
Methods: Two-cell embryos were isolated from stimulated mice. The embryos were grouped into "split" and "non-split" groups. The zona pellucida was removed from the split group and the blastomeres were distributed before being co-cultured with mouse embryo fibroblasts to the blastocyst stage. Normal (non-split) blastocysts were co-cultured in the same way. The 3.5-day-old blastomeres were collected as the control group. For molecular evaluation, real-time PCR was conducted to analyze changes in Cdx2, Sox2, Oct4, and Nanog gene expression. Moreover, the blastocyst formation rate, overall blastocyst rate, and the number of newborns were statistically analyzed.
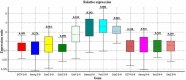
Results: The findings showed that embryo splitting increased blastocyst formation, overall blastocysts, developmental potential embryos, and the number of infants. Furthermore, the split and non-split (control) groups showed equal expression of pluripotent genes (Cdx2, Sox2, Oct4, and Nanog) in the molecular analysis.
Conclusion: It can be concluded that the growth and developmental potency of sister blastocysts derived from split two-cell stage mouse embryos are the same as those of normal blastocysts. So, there are no significant differences in gene expression between the split and non-split groups.
Keywords: Embryo splitting; Gene expression; Mouse blastocyst; Two-cell embryo.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Splitting of IVP bovine blastocyst affects morphology and gene expression of resulting demi-embryos during in vitro culture and in vivo elongation.Zygote. 2016 Feb;24(1):18-30. doi: 10.1017/S0967199414000677. Epub 2014 Dec 11. Zygote. 2016. PMID: 25496989
-
Domestic cat embryos generated without zona pellucida are capable of developing in vitro but exhibit abnormal gene expression and a decreased implantation rate.Theriogenology. 2021 Oct 15;174:36-46. doi: 10.1016/j.theriogenology.2021.08.013. Epub 2021 Aug 12. Theriogenology. 2021. PMID: 34416562
-
TEAD4 regulates trophectoderm differentiation upstream of CDX2 in a GATA3-independent manner in the human preimplantation embryo.Hum Reprod. 2022 Jul 30;37(8):1760-1773. doi: 10.1093/humrep/deac138. Hum Reprod. 2022. PMID: 35700449
-
In vitro blastocyst development from serially split mouse embryos and future implications for human assisted reproductive technologies.Fertil Steril. 2006 Oct;86(4 Suppl):1112-20. doi: 10.1016/j.fertnstert.2006.02.103. Epub 2006 Sep 7. Fertil Steril. 2006. PMID: 16962118 Review.
-
Retrospective analysis: reproducibility of interblastomere differences of mRNA expression in 2-cell stage mouse embryos is remarkably poor due to combinatorial mechanisms of blastomere diversification.Mol Hum Reprod. 2018 Jul 1;24(7):388-400. doi: 10.1093/molehr/gay021. Mol Hum Reprod. 2018. PMID: 29746690
Cited by
-
A comprehensive review: synergizing stem cell and embryonic development knowledge in mouse and human integrated stem cell-based embryo models.Front Cell Dev Biol. 2024 Apr 22;12:1386739. doi: 10.3389/fcell.2024.1386739. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38715920 Free PMC article. Review.
References
-
- Aktan E, Ozer D, Demirol A, Gurgan T, Bozkurt K. The effect of zona thinning size on implantation and pregnancy rates of ICSI-ET patients with advanced woman age. Middle East Fertil. Soc. J. 2006;11:183–190.
-
- Boiani M, Casser E, Fuellen G, Christians ES. Totipotency continuity from zygote to early blastomeres: a model under revision. Reproduction. 2019;158:R49–R65. - PubMed
-
- Casser E, Israel S, Boiani M. Multiplying embryos: experimental monozygotic polyembryony in mammals and its uses. Int. J. Dev. Biol. 2019;63:143–155. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
