Protocol Biopsy After Kidney Transplant: Clinical Application and Efficacy to Detect Allograft Rejection
- PMID: 36874304
- PMCID: PMC9983784
- DOI: 10.7759/cureus.34505
Protocol Biopsy After Kidney Transplant: Clinical Application and Efficacy to Detect Allograft Rejection
Abstract
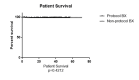
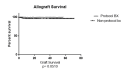
Background Kidney transplant rejection is a major cause of graft dysfunction and failure. In recent years, there has been increased interest in renal allograft protocol biopsies to allow earlier detection of acute or chronic graft dysfunction or rejection to improve long-term graft survival and reduce graft failure. This study aimed to determine if renal allograft protocol biopsies performed within the first 12 months after transplantation help detect subclinical graft dysfunction or rejection. Methods We performed a retrospective analysis utilizing SUNY Upstate University Hospital data from January 2016 to March 2022 to assess transplant outcomes and biopsies. The study population was divided into two subgroups: non-protocol biopsies and protocol biopsies within the 12 months post-transplant. Results A total of 332 patients met our inclusion criteria and were included in the study. Patients were divided into two subgroups: 135 patients (40.6%) in the protocol biopsy group and 197 patients (59.4%) with non-protocol indication biopsies during the first year after the transplant. The overall number of rejection episodes reported was eight episodes (4.6%) in the protocol biopsy group and 56 episodes (18.3%) in the non-protocol indication biopsy group, which was significantly higher in the non-protocol biopsy group (P=0.001). Antibody-mediated rejection (ABMR) and T-cell-mediated rejection (TCMR) diagnoses were significantly higher in the non-protocol biopsy group (P=0.03 and P=0.03, respectively). We also mentioned a trend in terms of mixed antibody-mediated rejection and T-cell-mediated rejection diagnosis (P=0.07). One year after the rejection, the mean glomerular filtration rate (GFR) was 56.78 mL/min/1.73m2 in the protocol biopsy group and 49.14 mL/min/1.73m2 in the non-protocol indication biopsy group, and there was no significant difference anymore (P=0.11). The patient survival rate was not significantly higher in the protocol biopsy group compared to the non-protocol indication biopsy group (P=0.42). Conclusion This study suggests that performing protocol biopsies does not significantly benefit rejection rates, graft survival, or renal function within the first 12 months post-transplant. Given these results and the small but non-zero risk of complications associated with protocol biopsies, they should be reserved for those patients at high risk of rejection. It may be more feasible and beneficial to utilize less invasive tests, such as DSA and dd-cfDNA testing, for early diagnosis of a rejection episode.
Keywords: dd-cfdna testing; kidney transplant; outcomes; protocol biopsy; rejection.
Copyright © 2023, Moein et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The Trifecta Study: Comparing Plasma Levels of Donor-derived Cell-Free DNA with the Molecular Phenotype of Kidney Transplant Biopsies.J Am Soc Nephrol. 2022 Feb;33(2):387-400. doi: 10.1681/ASN.2021091191. Epub 2022 Jan 20. J Am Soc Nephrol. 2022. PMID: 35058354 Free PMC article. Clinical Trial.
-
Microvascular inflammation in early protocol biopsies of renal allografts in cases of chronic active antibody-mediated rejection.Nephrology (Carlton). 2015 Jul;20 Suppl 2:26-30. doi: 10.1111/nep.12450. Nephrology (Carlton). 2015. PMID: 26031582
-
Utility of Serial Protocol Biopsies Performed After 1 Year in Predicting Long-Term Kidney Allograft Function According to Histologic Phenotype.Exp Clin Transplant. 2018 Aug;16(4):391-400. doi: 10.6002/ect.2016.0323. Epub 2017 Dec 5. Exp Clin Transplant. 2018. PMID: 29206090
-
Protocol Biopsies: Utility and Limitations.Adv Chronic Kidney Dis. 2016 Sep;23(5):326-331. doi: 10.1053/j.ackd.2016.09.002. Adv Chronic Kidney Dis. 2016. PMID: 27742388 Review.
-
Does subclinical rejection contribute to chronic rejection in renal transplant patients?Clin Transplant. 1999 Dec;13(6):441-6. doi: 10.1034/j.1399-0012.1999.130601.x. Clin Transplant. 1999. PMID: 10617231 Review.
Cited by
-
Hyperspectral imaging in living and deceased donor kidney transplantation.BMC Med Imaging. 2025 Jan 31;25(1):34. doi: 10.1186/s12880-025-01576-6. BMC Med Imaging. 2025. PMID: 39891083 Free PMC article.
-
Protocol Biopsies in Kidney Transplant Recipients: Current Practice After Much Discussion.Biomedicines. 2025 Jul 7;13(7):1660. doi: 10.3390/biomedicines13071660. Biomedicines. 2025. PMID: 40722731 Free PMC article. Review.
-
Diagnostic performance of GcfDNA in kidney allograft rejection: a meta-analysis.Front Physiol. 2024 Jan 9;14:1293402. doi: 10.3389/fphys.2023.1293402. eCollection 2023. Front Physiol. 2024. PMID: 38264334 Free PMC article.
-
Follow-up biopsies with microvascular inflammation and persistent donor specific antibodies identify ongoing rejection in pediatric kidney transplant recipients.Pediatr Nephrol. 2025 Jul;40(7):2375-2382. doi: 10.1007/s00467-025-06671-y. Epub 2025 Jan 28. Pediatr Nephrol. 2025. PMID: 39873804
-
Predicting Cellular Rejection of Renal Allograft Based on the Serum Proteomic Fingerprint.Int J Mol Sci. 2024 Mar 29;25(7):3844. doi: 10.3390/ijms25073844. Int J Mol Sci. 2024. PMID: 38612654 Free PMC article.
References
-
- Evaluation of adult kidney transplant candidates. Bunnapradist S, Danovitch GM. Am J Kidney Dis. 2007;50:890–898. - PubMed
-
- Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Tonelli M, Wiebe N, Knoll G, et al. Am J Transplant. 2011;11:2093–2109. - PubMed
-
- Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Meier-Kriesche HU, Kaplan B. Transplantation. 2002;74:1377–1381. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous