Deep learning-enabled volumetric cone photoreceptor segmentation in adaptive optics optical coherence tomography images of normal and diseased eyes
- PMID: 36874491
- PMCID: PMC9979662
- DOI: 10.1364/BOE.478693
Deep learning-enabled volumetric cone photoreceptor segmentation in adaptive optics optical coherence tomography images of normal and diseased eyes
Abstract
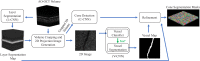
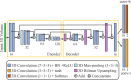
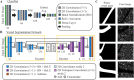
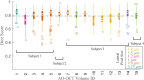
Objective quantification of photoreceptor cell morphology, such as cell diameter and outer segment length, is crucial for early, accurate, and sensitive diagnosis and prognosis of retinal neurodegenerative diseases. Adaptive optics optical coherence tomography (AO-OCT) provides three-dimensional (3-D) visualization of photoreceptor cells in the living human eye. The current gold standard for extracting cell morphology from AO-OCT images involves the tedious process of 2-D manual marking. To automate this process and extend to 3-D analysis of the volumetric data, we propose a comprehensive deep learning framework to segment individual cone cells in AO-OCT scans. Our automated method achieved human-level performance in assessing cone photoreceptors of healthy and diseased participants captured with three different AO-OCT systems representing two different types of point scanning OCT: spectral domain and swept source.
© 2023 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the US Department of Health and Human Services.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
