Risk factor-based screening compared to universal screening for gestational diabetes mellitus in marginalized Burman and Karen populations on the Thailand-Myanmar border: An observational cohort
- PMID: 36874585
- PMCID: PMC9976631
- DOI: 10.12688/wellcomeopenres.17743.2
Risk factor-based screening compared to universal screening for gestational diabetes mellitus in marginalized Burman and Karen populations on the Thailand-Myanmar border: An observational cohort
Abstract
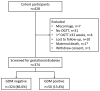
Background: Gestational diabetes mellitus (GDM) contributes to maternal and neonatal morbidity. As data from marginalized populations remains scarce, this study compares risk-factor-based to universal GDM screening in a low resource setting. Methods: This is a secondary analysis of data from a prospective preterm birth cohort. Pregnant women were enrolled in the first trimester and completed a 75g oral glucose tolerance test (OGTT) at 24-32 weeks' gestation. To define GDM cases, Hyperglycaemia and Adverse Pregnancy Outcomes (HAPO trial) criteria were used. All GDM positive cases were treated. Sensitivity and specificity of risk-factor-based selection for screening (criteria: age ≥30y, obesity (Body mass index (BMI) ≥27.5kg/m 2), previous GDM, 1 st degree relative with diabetes, previous macrosomia (≥4kg), previous stillbirth, or symphysis-fundal height ≥90th percentile) was compared to universal screening using the OGTT as the gold standard. Adverse maternal and neonatal outcomes were compared by GDM status. Results: GDM prevalence was 13.4% (50/374) (95% CI: 10.3-17.2). Three quarters of women had at least one risk factor (n=271 women), with 37/50 OGTT positive cases correctly identified: sensitivity 74.0% (59.7-85.4) and specificity 27.8% (3.0-33.0). Burman women (self-identified) accounted for 29.1% of the cohort population, but 38.0% of GDM cases. Percentiles for birthweight (p=0.004), head circumference (p=0.002), and weight-length ratio (p=0.030) were higher in newborns of GDM positive compared with non-GDM mothers. 21.7% (75/346) of newborns in the cohort were small-for-gestational age (≤10 th percentile). In Burman women, overweight/obese BMI was associated with a significantly increased adjusted odds ratio 5.03 (95% CI: 1.43-17.64) for GDM compared with normal weight, whereas in Karen women, the trend in association was similar but not significant (OR 2.36; 95% CI 0.95-5.89). Conclusions: Risk-factor-based screening missed one in four GDM positive women. Considering the benefits of early detection of GDM and the limited additional cost of universal screening, a two-step screening program was implemented.
Keywords: Gestational diabetes mellitus; HAPO trial; Maternal and neonatal anthropometry; Migrants; Oral glucose tolerance test; Risk-factor-based screening; Symphysis-fundal height measurements; thin-diabetic.
Copyright: © 2023 Prüst JT et al.
Conflict of interest statement
No competing interests were disclosed.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

