Determining the Optimal SARS-CoV-2 mRNA Vaccine Dosing Interval for Maximum Immunogenicity
- PMID: 36874687
- PMCID: PMC9981229
- DOI: 10.7759/cureus.34465
Determining the Optimal SARS-CoV-2 mRNA Vaccine Dosing Interval for Maximum Immunogenicity
Abstract
Objective: Emerging evidence indicates that longer SARS-CoV-2 vaccine dosing intervals results in an enhanced immune response. However, the optimal vaccine dosing interval for achieving maximum immunogenicity is unclear.
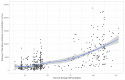
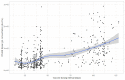
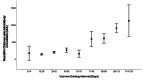
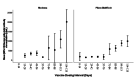
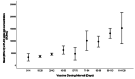
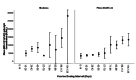
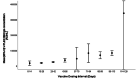
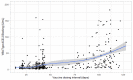
Methods: This study included samples from adult paramedics in Canada who received two doses of either BNT162b2 or mRNA-1273 vaccines and provided blood samples six months (170 to 190 days) after the first vaccine dose. The main exposure variable was vaccine dosing interval (days), categorized as "short" (first quartile), "moderate" (second quartile), "long" (third quartile), and "longest" interval (fourth quartile). The primary outcome was total spike antibody concentrations, measured using the Elecsys SARS-CoV-2 total antibody assay. Secondary outcomes included spike and receptor-binding domain (RBD) immunoglobulin G (IgG) antibody concentrations, and inhibition of angiotensin-converting enzyme 2 (ACE-2) binding to wild-type spike protein and several different Delta variant spike proteins. We fit a multiple log-linear regression model to investigate the association between vaccine dosing intervals and the antibody concentrations.
Results: A total of 564 adult paramedics (mean age 40 years, SD=10) were included. Compared to "short interval" (≤30 days), vaccine dosing intervals of the long (39-73 days) group (β= 0.31, 95% Confidence interval (CI): 0.10-0.52) and the longest (≥74 days) group (β = 0.82. 95% CI: 0.36-1.28) were associated with increased spike total antibody concentration. Compared to the short interval, the longest interval quartile was associated with higher spike IgG antibodies, while the long and longest intervals were associated with higher RBD IgG antibody concentrations. Similarly, the longest dosing intervals increased inhibition of ACE-2 binding to viral spike protein.
Conclusion: Increased mRNA vaccine dosing intervals longer than 38 days result in higher levels of anti-spike antibodies and ACE-2 inhibition when assessed six months after the first COVID-19 vaccine.
Keywords: covid-19; covid-19 mrna vaccine; immunogenicity; sars-cov-2; spike total antibody concentrations; vaccine dosing interval.
Copyright © 2023, Asamoah-Boaheng et al.
Conflict of interest statement
The authors have declared financial relationships, which are detailed in the next section.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous
