Ongoing involvers and promising therapeutic targets of hepatic fibrosis: The hepatic immune microenvironment
- PMID: 36875101
- PMCID: PMC9978172
- DOI: 10.3389/fimmu.2023.1131588
Ongoing involvers and promising therapeutic targets of hepatic fibrosis: The hepatic immune microenvironment
Abstract
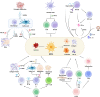
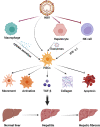
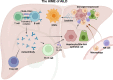
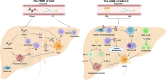
Hepatic fibrosis is often secondary to chronic inflammatory liver injury. During the development of hepatic fibrosis, the damaged hepatocytes and activated hepatic stellate cells (HSCs) caused by the pathogenic injury could secrete a variety of cytokines and chemokines, which will chemotactic innate and adaptive immune cells of liver tissue and peripheral circulation infiltrating into the injury site, mediating the immune response against injury and promoting tissue reparation. However, the continuous release of persistent injurious stimulus-induced inflammatory cytokines will promote HSCs-mediated fibrous tissue hyperproliferation and excessive repair, which will cause hepatic fibrosis development and progression to cirrhosis even liver cancer. And the activated HSCs can secrete various cytokines and chemokines, which directly interact with immune cells and actively participate in liver disease progression. Therefore, analyzing the changes in local immune homeostasis caused by immune response under different pathological states will greatly enrich our understanding of liver diseases' reversal, chronicity, progression, and even deterioration of liver cancer. In this review, we summarized the critical components of the hepatic immune microenvironment (HIME), different sub-type immune cells, and their released cytokines, according to their effect on the development of progression of hepatic fibrosis. And we also reviewed and analyzed the specific changes and the related mechanisms of the immune microenvironment in different chronic liver diseases.Moreover, we retrospectively analyzed whether the progression of hepatic fibrosis could be alleviated by modulating the HIME.We aimed to elucidate the pathogenesis of hepatic fibrosis and provide the possibility for exploring the therapeutic targets for hepatic fibrosis.
Keywords: chronic liver diseases; hepatic fibrosis; hepatic immune microenvironment; hepatic stellate cells (HSCs); immune cells.
Copyright © 2023 Zhang, Yao, Zhang, Li, Chen, Zhao, Ju, He, Pan, Liu and Lv.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

