Understanding disruption of the gut barrier during inflammation: Should we abandon traditional epithelial cell lines and switch to intestinal organoids?
- PMID: 36875103
- PMCID: PMC9983034
- DOI: 10.3389/fimmu.2023.1108289
Understanding disruption of the gut barrier during inflammation: Should we abandon traditional epithelial cell lines and switch to intestinal organoids?
Abstract
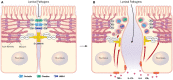
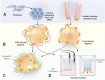
Disruption of the intestinal epithelial barrier is a hallmark of mucosal inflammation. It increases exposure of the immune system to luminal microbes, triggering a perpetuating inflammatory response. For several decades, the inflammatory stimuli-induced breakdown of the human gut barrier was studied in vitro by using colon cancer derived epithelial cell lines. While providing a wealth of important data, these cell lines do not completely mimic the morphology and function of normal human intestinal epithelial cells (IEC) due to cancer-related chromosomal abnormalities and oncogenic mutations. The development of human intestinal organoids provided a physiologically-relevant experimental platform to study homeostatic regulation and disease-dependent dysfunctions of the intestinal epithelial barrier. There is need to align and integrate the emerging data obtained with intestinal organoids and classical studies that utilized colon cancer cell lines. This review discusses the utilization of human intestinal organoids to dissect the roles and mechanisms of gut barrier disruption during mucosal inflammation. We summarize available data generated with two major types of organoids derived from either intestinal crypts or induced pluripotent stem cells and compare them to the results of earlier studies with conventional cell lines. We identify research areas where the complementary use of colon cancer-derived cell lines and organoids advance our understanding of epithelial barrier dysfunctions in the inflamed gut and identify unique questions that could be addressed only by using the intestinal organoid platforms.
Keywords: actin cytoskeleton; adherens junctions; colonoids; cytokines; enteroids; epithelial barrier; inflammatory bowel diseases; tight junctions.
Copyright © 2023 Lechuga, Braga-Neto, Naydenov, Rieder and Ivanov.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Loss of β-Cytoplasmic Actin in the Intestinal Epithelium Increases Gut Barrier Permeability in vivo and Exaggerates the Severity of Experimental Colitis.Front Cell Dev Biol. 2020 Oct 23;8:588836. doi: 10.3389/fcell.2020.588836. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195251 Free PMC article.
-
The application of explants, crypts, and organoids as models in intestinal barrier research.Tissue Barriers. 2024 Nov 5:2423137. doi: 10.1080/21688370.2024.2423137. Online ahead of print. Tissue Barriers. 2024. PMID: 39499114
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
A Novel Microphysiological Colon Platform to Decipher Mechanisms Driving Human Intestinal Permeability.Cell Mol Gastroenterol Hepatol. 2021;12(5):1719-1741. doi: 10.1016/j.jcmgh.2021.07.004. Epub 2021 Jul 17. Cell Mol Gastroenterol Hepatol. 2021. PMID: 34284165 Free PMC article.
-
Intestinal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases.World J Gastroenterol. 2019 Aug 14;25(30):4125-4147. doi: 10.3748/wjg.v25.i30.4125. World J Gastroenterol. 2019. PMID: 31435168 Free PMC article. Review.
Cited by
-
Claudin Barriers on the Brink: How Conflicting Tissue and Cellular Priorities Drive IBD Pathogenesis.Int J Mol Sci. 2023 May 10;24(10):8562. doi: 10.3390/ijms24108562. Int J Mol Sci. 2023. PMID: 37239907 Free PMC article. Review.
-
Research progress of autoimmune diseases based on induced pluripotent stem cells.Front Immunol. 2024 Apr 24;15:1349138. doi: 10.3389/fimmu.2024.1349138. eCollection 2024. Front Immunol. 2024. PMID: 38720903 Free PMC article. Review.
-
A short guide to the tight junction.J Cell Sci. 2024 May 1;137(9):jcs261776. doi: 10.1242/jcs.261776. Epub 2024 May 7. J Cell Sci. 2024. PMID: 38712627 Free PMC article. Review.
-
Mechanism of cell death and its application in the repair of inflammatory bowel disease by mesenchymal stem cells.Front Immunol. 2025 Jun 4;16:1597462. doi: 10.3389/fimmu.2025.1597462. eCollection 2025. Front Immunol. 2025. PMID: 40534879 Free PMC article. Review.
-
Role of Extracellular Vesicles in Crohn's Patients on Adalimumab Who Received COVID-19 Vaccination.Int J Mol Sci. 2024 Aug 14;25(16):8853. doi: 10.3390/ijms25168853. Int J Mol Sci. 2024. PMID: 39201543 Free PMC article.
References
-
- Rubsam M, Broussard JA, Wickstrom SA, Nekrasova O, Green KJ, Niessen CM. Adherens junctions and desmosomes coordinate mechanics and signaling to orchestrate tissue morphogenesis and function: An evolutionary perspective. Cold Spring Harb Perspect Biol (2018) 10:a029207. doi: 10.1101/cshperspect.a029207 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

