Therapeutic equine hyperimmune antibodies with high and broad-spectrum neutralizing activity protect rodents against SARS-CoV-2 infection
- PMID: 36875106
- PMCID: PMC9981790
- DOI: 10.3389/fimmu.2023.1066730
Therapeutic equine hyperimmune antibodies with high and broad-spectrum neutralizing activity protect rodents against SARS-CoV-2 infection
Abstract
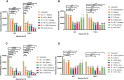
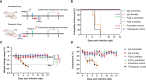
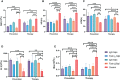
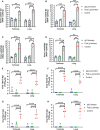
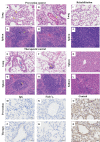
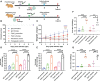
The emergence of SARS-CoV-2 variants stresses the continued need for broad-spectrum therapeutic antibodies. Several therapeutic monoclonal antibodies or cocktails have been introduced for clinical use. However, unremitting emerging SARS-CoV-2 variants showed reduced neutralizing efficacy by vaccine induced polyclonal antibodies or therapeutic monoclonal antibodies. In our study, polyclonal antibodies and F(ab')2 fragments with strong affinity produced after equine immunization with RBD proteins produced strong affinity. Notably, specific equine IgG and F(ab')2 have broad and high neutralizing activity against parental virus, all SARS-CoV-2 variants of concern (VOCs), including B.1.1,7, B.1.351, B.1.617.2, P.1, B.1.1.529 and BA.2, and all variants of interest (VOIs) including B.1.429, P.2, B.1.525, P.3, B.1.526, B.1.617.1, C.37 and B.1.621. Although some variants weaken the neutralizing ability of equine IgG and F(ab')2 fragments, they still exhibited superior neutralization ability against mutants compared to some reported monoclonal antibodies. Furthermore, we tested the pre-exposure and post-exposure protective efficacy of the equine immunoglobulin IgG and F(ab')2 fragments in lethal mouse and susceptible golden hamster models. Equine immunoglobulin IgG and F(ab')2 fragments effectively neutralized SARS-CoV-2 in vitro, fully protected BALB/c mice from the lethal challenge, and reduced golden hamster's lung pathological change. Therefore, equine pAbs are an adequate, broad coverage, affordable and scalable potential clinical immunotherapy for COVID-19, particularly for SARS-CoV-2 VOCs or VOIs.
Keywords: SARS-CoV-2; broad-spectrum neutralizing activity; equine antibody; variants of concern; variants of interest.
Copyright © 2023 Li, Han, Bi, Wei, Wang, Zhang, Liu, Feng, Wang, Wu, Yang, Zhao, Liu, Yan and Xia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
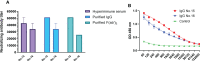
Similar articles
-
Equine immunoglobulin fragment F(ab')2 displays high neutralizing capability against multiple SARS-CoV-2 variants.Clin Immunol. 2022 Apr;237:108981. doi: 10.1016/j.clim.2022.108981. Epub 2022 Mar 17. Clin Immunol. 2022. PMID: 35306171 Free PMC article.
-
Passive immunization with equine RBD-specific Fab protects K18-hACE2-mice against Alpha or Beta variants of SARS-CoV-2.Front Immunol. 2022 Aug 15;13:948431. doi: 10.3389/fimmu.2022.948431. eCollection 2022. Front Immunol. 2022. PMID: 36091051 Free PMC article.
-
Potent Neutralizing Activity of Polyclonal Equine Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 Variants of Concern.J Infect Dis. 2022 Dec 28;227(1):35-39. doi: 10.1093/infdis/jiac331. J Infect Dis. 2022. PMID: 35921532 Free PMC article.
-
Transchromosomic bovines-derived broadly neutralizing antibodies as potent biotherapeutics to counter important emerging viral pathogens with a special focus on SARS-CoV-2, MERS-CoV, Ebola, Zika, HIV-1, and influenza A virus.J Med Virol. 2022 Oct;94(10):4599-4610. doi: 10.1002/jmv.27907. Epub 2022 Jun 11. J Med Virol. 2022. PMID: 35655326 Free PMC article. Review.
-
Function and mechanism of bispecific antibodies targeting SARS-CoV-2.Cell Insight. 2024 Feb 3;3(2):100150. doi: 10.1016/j.cellin.2024.100150. eCollection 2024 Apr. Cell Insight. 2024. PMID: 38374826 Free PMC article. Review.
Cited by
-
SARS-CoV-2 ferritin nanoparticle vaccines produce hyperimmune equine sera with broad sarbecovirus activity.iScience. 2024 Aug 23;27(10):110624. doi: 10.1016/j.isci.2024.110624. eCollection 2024 Oct 18. iScience. 2024. PMID: 39351195 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

