Development and validation of a radiomics-based nomogram for predicting a major pathological response to neoadjuvant immunochemotherapy for patients with potentially resectable non-small cell lung cancer
- PMID: 36875128
- PMCID: PMC9978193
- DOI: 10.3389/fimmu.2023.1115291
Development and validation of a radiomics-based nomogram for predicting a major pathological response to neoadjuvant immunochemotherapy for patients with potentially resectable non-small cell lung cancer
Abstract
Introduction: The treatment response to neoadjuvant immunochemotherapy varies among patients with potentially resectable non-small cell lung cancers (NSCLC) and may have severe immune-related adverse effects. We are currently unable to accurately predict therapeutic response. We aimed to develop a radiomics-based nomogram to predict a major pathological response (MPR) of potentially resectable NSCLC to neoadjuvant immunochemotherapy using pretreatment computed tomography (CT) images and clinical characteristics.
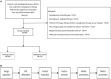
Methods: A total of 89 eligible participants were included and randomly divided into training (N=64) and validation (N=25) sets. Radiomic features were extracted from tumor volumes of interest in pretreatment CT images. Following data dimension reduction, feature selection, and radiomic signature building, a radiomics-clinical combined nomogram was developed using logistic regression analysis.
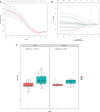
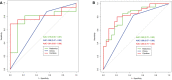
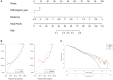
Results: The radiomics-clinical combined model achieved excellent discriminative performance, with AUCs of 0.84 (95% CI, 0.74-0.93) and 0.81(95% CI, 0.63-0.98) and accuracies of 80% and 80% in the training and validation sets, respectively. Decision curves analysis (DCA) indicated that the radiomics-clinical combined nomogram was clinically valuable.
Discussion: The constructed nomogram was able to predict MPR to neoadjuvant immunochemotherapy with a high degree of accuracy and robustness, suggesting that it is a convenient tool for assisting with the individualized management of patients with potentially resectable NSCLC.
Keywords: NSCLC; major pathological response; neoadjuvant immunochemotherapy; nomogram; radiomics.
Copyright © 2023 Liu, Zhao, Xie, Lin, Hu, Li, Shang, Wang, Jiang, Ding, Peng, Xu, Hu, Huang, Gao, Liu, Liu and Ma.
Conflict of interest statement
Author HL was employed by the company GE Healthcare. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. . The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol (2016) 11(1):39–51. doi: 10.1016/j.jtho.2015.09.009 - DOI - PubMed
-
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. . The iaslc lung cancer staging project: Proposals for revision of the tnm stage groupings in the forthcoming (Eighth) edition of the tnm classification for lung cancer. J Thorac Oncol (2016) 11(1):39–51. doi: 10.1016/j.jtho.2015.09.009 - DOI - PubMed
-
- Hellmann MD, Chaft JE, William WN, Rusch V, Pisters KMW, Kalhor N, et al. . Pathological response after neoadjuvant chemotherapy in resectable non-Small-Cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol (2014) 15(1):e42–50. doi: 10.1016/s1470-2045(13)70334-6 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

