CD83 expressed by macrophages is an important immune checkpoint molecule for the resolution of inflammation
- PMID: 36875129
- PMCID: PMC9975560
- DOI: 10.3389/fimmu.2023.1085742
CD83 expressed by macrophages is an important immune checkpoint molecule for the resolution of inflammation
Abstract
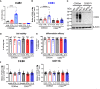
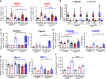
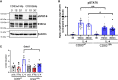
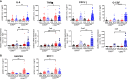
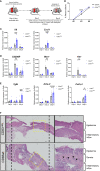
Excessive macrophage (Mφ) activation results in chronic inflammatory responses or autoimmune diseases. Therefore, identification of novel immune checkpoints on Mφ, which contribute to resolution of inflammation, is crucial for the development of new therapeutic agents. Herein, we identify CD83 as a marker for IL-4 stimulated pro-resolving alternatively activated Mφ (AAM). Using a conditional KO mouse (cKO), we show that CD83 is important for the phenotype and function of pro-resolving Mφ. CD83-deletion in IL-4 stimulated Mφ results in decreased levels of inhibitory receptors, such as CD200R and MSR-1, which correlates with a reduced phagocytic capacity. In addition, CD83-deficient Mφ upon IL-4 stimulation, show an altered STAT-6 phosphorylation pattern, which is characterized by reduced pSTAT-6 levels and expression of the target gene Gata3. Concomitantly, functional studies in IL-4 stimulated CD83 KO Mφ reveal an increased production of pro-inflammatory mediators, such as TNF-α, IL-6, CXCL1 and G-CSF. Furthermore, we show that CD83-deficient Mφ have enhanced capacities to stimulate the proliferation of allo-reactive T cells, which was accompanied by reduced frequencies of Tregs. In addition, we show that CD83 expressed by Mφ is important to limit the inflammatory phase using a full-thickness excision wound healing model, since inflammatory transcripts (e.g. Cxcl1, Il6) were increased, whilst resolving transcripts (e.g. Ym1, Cd200r, Msr-1) were decreased in wounds at day 3 after wound infliction, which reflects the CD83 resolving function on Mφ also in vivo. Consequently, this enhanced inflammatory milieu led to an altered tissue reconstitution after wound infliction. Thus, our data provide evidence that CD83 acts as a gatekeeper for the phenotype and function of pro-resolving Mφ.
Keywords: CD83; STAT-6; checkpoint molecule; macrophages; resolution of inflammation; wound healing.
Copyright © 2023 Peckert-Maier, Langguth, Strack, Stich, Mühl-Zürbes, Kuhnt, Drassner, Zinser, Wrage, Mattner, Steinkasserer, Royzman and Wild.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Soluble CD83 modulates human-monocyte-derived macrophages toward alternative phenotype, function, and metabolism.Front Immunol. 2023 Dec 14;14:1293828. doi: 10.3389/fimmu.2023.1293828. eCollection 2023. Front Immunol. 2023. PMID: 38162675 Free PMC article.
-
Tilting the Balance: Therapeutic Prospects of CD83 as a Checkpoint Molecule Controlling Resolution of Inflammation.Int J Mol Sci. 2022 Jan 10;23(2):732. doi: 10.3390/ijms23020732. Int J Mol Sci. 2022. PMID: 35054916 Free PMC article. Review.
-
CD83 acts as immediate early response gene in activated macrophages and exhibits specific intracellular trafficking properties.Biochem Biophys Res Commun. 2023 Mar 5;647:37-46. doi: 10.1016/j.bbrc.2023.01.069. Epub 2023 Jan 23. Biochem Biophys Res Commun. 2023. PMID: 36709671
-
IL-4 reduces the proangiogenic capacity of macrophages by down-regulating HIF-1α translation.J Leukoc Biol. 2014 Jan;95(1):129-37. doi: 10.1189/jlb.0113045. Epub 2013 Sep 4. J Leukoc Biol. 2014. PMID: 24006507
-
Regulation of Macrophage Polarization and Wound Healing.Adv Wound Care (New Rochelle). 2012 Feb;1(1):10-16. doi: 10.1089/wound.2011.0307. Adv Wound Care (New Rochelle). 2012. PMID: 24527272 Free PMC article. Review.
Cited by
-
Therapeutic Properties of M2 Macrophages in Chronic Wounds: An Innovative Area of Biomaterial-Assisted M2 Macrophage Targeted Therapy.Stem Cell Rev Rep. 2025 Feb;21(2):390-422. doi: 10.1007/s12015-024-10806-3. Epub 2024 Nov 18. Stem Cell Rev Rep. 2025. PMID: 39556244 Review.
-
Transcriptomic profiling after B cell depletion reveals central and peripheral immune cell changes in multiple sclerosis.J Clin Invest. 2025 Mar 11;135(11):e182790. doi: 10.1172/JCI182790. eCollection 2025 Jun 2. J Clin Invest. 2025. PMID: 40067358 Free PMC article.
-
Reduced tolerogenic factor sCD83 in NMOSD and relapsing MOGAD: a potential new therapeutic pathway.Front Immunol. 2025 Jul 24;16:1620069. doi: 10.3389/fimmu.2025.1620069. eCollection 2025. Front Immunol. 2025. PMID: 40777014 Free PMC article.
-
Transcriptomic Responses to Koi Herpesvirus in Isolated Blood Leukocytes from Infected Common Carp.Viruses. 2024 Feb 28;16(3):380. doi: 10.3390/v16030380. Viruses. 2024. PMID: 38543746 Free PMC article.
-
Lung Single-Cell Transcriptomics in Alpha-1 Antitrypsin Deficiency.Am J Respir Cell Mol Biol. 2024 Aug;71(2):254-256. doi: 10.1165/rcmb.2024-0064LE. Am J Respir Cell Mol Biol. 2024. PMID: 39087828 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

