Regulatory T cell enhancement in adults with cystic fibrosis receiving Elexacaftor/Tezacaftor/Ivacaftor therapy
- PMID: 36875141
- PMCID: PMC9978140
- DOI: 10.3389/fimmu.2023.1107437
Regulatory T cell enhancement in adults with cystic fibrosis receiving Elexacaftor/Tezacaftor/Ivacaftor therapy
Abstract
Introduction: Cystic fibrosis (CF), especially CF lung disease, is characterized by chronic infection, immune dysfunction including impairment of regulatory T cells (Tregs) and an exaggerated inflammatory response. CF transmembrane conductance regulator (CFTR) modulators have shown to improve clinical outcomes in people with CF (PwCF) with a wide range of CFTR mutations. However, it remains unclear whether CFTR modulator therapy also affects CF-associated inflammation. We aimed to examine the effect of elexacaftor/tezacaftor/ivacaftor therapy on lymphocyte subsets and systemic cytokines in PwCF.
Methods: Peripheral blood mononuclear cells and plasma were collected before and at three and six months after the initiation of elexacaftor/tezacaftor/ivacaftor therapy; lymphocyte subsets and systemic cytokines were determined using flow cytometry.
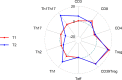
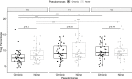
Results: Elexacaftor/tezacaftor/ivacaftor treatment was initiated in 77 PwCF and improved percent predicted FEV1 by 12.5 points (p<0.001) at 3 months. During elexacaftor/tezacaftor/ivacaftor therapy, percentages of Tregs were enhanced (+18.7%, p<0.001), with an increased proportion of Tregs expressing CD39 as a marker of stability (+14.4%, p<0.001). Treg enhancement was more pronounced in PwCF clearing Pseudomonas aeruginosa infection. Only minor, non-significant shifts were observed among Th1-, Th2- and Th17-expressing effector T helper cells. These results were stable at 3- and 6-month follow-up. Cytokine measurements showed a significant decrease in interleukin-6 levels during treatment with elexacaftor/tezacaftor/ivacaftor (-50.2%, p<0.001).
Conclusion: Treatment with elexacaftor/tezacaftor/ivacaftor was associated with an increased percentage of Tregs, especially in PwCF clearing Pseudomonas aeruginosa infection. Targeting Treg homeostasis is a therapeutic option for PwCF with persistent Treg impairment.
Keywords: CFTR modulator therapy; Pseudomonas aeruginosa; cystic fibrosis - immunology; cytokines; immunophenotyping; pulmonary infection.
Copyright © 2023 Westhölter, Raspe, Uebner, Pipping, Schmitz, Straßburg, Sutharsan, Welsner, Taube and Reuter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Pseudomonas aeruginosa infection, but not mono or dual-combination CFTR modulator therapy affects circulating regulatory T cells in an adult population with cystic fibrosis.J Cyst Fibros. 2021 Nov;20(6):1072-1079. doi: 10.1016/j.jcf.2021.05.001. Epub 2021 May 21. J Cyst Fibros. 2021. PMID: 34030985
-
Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: a 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial.Lancet Respir Med. 2022 Mar;10(3):267-277. doi: 10.1016/S2213-2600(21)00454-9. Epub 2021 Dec 20. Lancet Respir Med. 2022. PMID: 34942085 Clinical Trial.
-
Analyzing the use and impact of elexacaftor/tezacaftor/ivacaftor on total cost of care and other health care resource utilization in a commercially insured population.J Manag Care Spec Pharm. 2022 Jul;28(7):721-731. doi: 10.18553/jmcp.2022.28.7.721. J Manag Care Spec Pharm. 2022. PMID: 35737861 Free PMC article.
-
Elexacaftor/Ivacaftor/Tezacaftor: First Approval.Drugs. 2019 Dec;79(18):2001-2007. doi: 10.1007/s40265-019-01233-7. Drugs. 2019. PMID: 31784874 Review.
-
Managing cystic fibrosis in children aged 6-11yrs: a critical review of elexacaftor/tezacaftor/ivacaftor combination therapy.Expert Rev Respir Med. 2023 Feb;17(2):97-108. doi: 10.1080/17476348.2023.2179989. Epub 2023 Feb 26. Expert Rev Respir Med. 2023. PMID: 36803356 Review.
Cited by
-
Aspergillus in Children and Young People with Cystic Fibrosis: A Narrative Review.J Fungi (Basel). 2025 Mar 9;11(3):210. doi: 10.3390/jof11030210. J Fungi (Basel). 2025. PMID: 40137248 Free PMC article. Review.
-
Modulator-refractory cystic fibrosis: Defining the scope and challenges of an emerging at-risk population.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241297877. doi: 10.1177/17534666241297877. Ther Adv Respir Dis. 2024. PMID: 39543951 Free PMC article. Review.
-
Pseudomonas aeruginosa in chronic lung disease: untangling the dysregulated host immune response.Front Immunol. 2024 Jun 28;15:1405376. doi: 10.3389/fimmu.2024.1405376. eCollection 2024. Front Immunol. 2024. PMID: 39015565 Free PMC article. Review.
-
Neoadjuvant nivolumab with or without relatlimab in resectable non-small-cell lung cancer: a randomized phase 2 trial.Nat Med. 2024 Jun;30(6):1602-1611. doi: 10.1038/s41591-024-02965-0. Epub 2024 Apr 30. Nat Med. 2024. PMID: 38689060 Free PMC article. Clinical Trial.
-
Anti-inflammatory effects of elexacaftor/tezacaftor/ivacaftor in adults with cystic fibrosis heterozygous for F508del.PLoS One. 2024 May 31;19(5):e0304555. doi: 10.1371/journal.pone.0304555. eCollection 2024. PLoS One. 2024. PMID: 38820269 Free PMC article.
References
-
- Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. . Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet (2019) 394:1940–8. doi: 10.1016/S0140-6736(19)32597-8 - DOI - PMC - PubMed
-
- Matouk E, Nguyen D, Benedetti A, Bernier J, Gruber J, Landry J, et al. . C-reactive protein in stable cystic fibrosis: an additional indicator of clinical disease activity and risk of future pulmonary exacerbations. J Pulm Respir Med (2016) 6:1000375. doi: 10.4172/2161-105X.1000375 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

