Impact of 6-month triptorelin formulation on predicted adult height and basal gonadotropin levels in patients with central precocious puberty
- PMID: 36875449
- PMCID: PMC9982112
- DOI: 10.3389/fendo.2023.1134977
Impact of 6-month triptorelin formulation on predicted adult height and basal gonadotropin levels in patients with central precocious puberty
Abstract
Background: Triptorelin, a long-acting gonadotropin-releasing hormone (GnRH) agonist, is available in 1-, 3-, and 6-month formulations to treat central precocious puberty (CPP). The triptorelin pamoate 22.5-mg 6-month formulation recently approved for CPP offers greater convenience to children by reducing the injection frequency. However, worldwide research on using the 6-month formulation to treat CPP is scarce. This study aimed to determine the impact of the 6-month formulation on predicted adult height (PAH), changes in gonadotropin levels, and related variables.
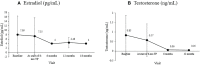
Methods: We included 42 patients (33 girls and nine boys) with idiopathic CPP treated with a 6-month triptorelin (6-mo TP) formulation for over 12 months. Auxological parameters, including chronological age, bone age, height (cm and standard deviation score [SDS]), weight (kg and SDS), target height (TH), and Tanner stage, were evaluated at baseline, and after 6, 12, and 18 months of treatment. Hormonal parameters, including serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), and estradiol for girls or testosterone for boys, were analyzed concurrently.
Results: The mean age at treatment initiation was 8.6 ± 0.83 (8.3 ± 0.62 for girls, 9.6 ± 0.68 for boys). The peak LH level following intravenous GnRH stimulation at diagnosis was 15.47 ± 9.94 IU/L. No progression of the modified Tanner stage was observed during treatment. Compared to baseline, LH, FSH, estradiol, and testosterone were significantly reduced. In particular, the basal LH levels were well suppressed to less than l.0 IU/L, and the LH/FSH ratio was less than 0.66. The bone age/chronological age ratio remained stable with a decreasing trend (1.15 at the start of treatment, 1.13 at 12 months, 1.11 at 18 months). PAH SDS increased during treatment (0.77 ± 0.79 at baseline, 0.87 ± 0.84 at the start of treatment, 1.01 ± 0.93 at six months, and 0.91 ± 0.79 at 12 months). No adverse effects were observed during treatment.
Conclusion: The 6-mo TP suppressed the pituitary-gonadal axis stably and improved the PAH during treatment. Considering its convenience and effectiveness, a significant shift to long-acting formulations can be expected.
Keywords: 6-month formulation; central precocious puberty; gonadotrophin-releasing hormone analogue; gonadotropins; triptorelin pamoate.
Copyright © 2023 Yoo, Kim, Jung, Shim, Shim, Kim, Kwak, Kim and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A Phase 3, Open-Label, Single-Arm Trial of the Efficacy and Safety of Triptorelin 6-Month Formulation in Chinese Children with Central Precocious Puberty.Adv Ther. 2024 Dec;41(12):4537-4556. doi: 10.1007/s12325-024-02991-x. Epub 2024 Oct 16. Adv Ther. 2024. PMID: 39412628 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Triptorelin 3-Month Formulation in Chinese Children with Central Precocious Puberty: A Phase 3, Open-Label, Single-Arm Study.Adv Ther. 2023 Oct;40(10):4574-4588. doi: 10.1007/s12325-023-02617-8. Epub 2023 Aug 16. Adv Ther. 2023. PMID: 37584898 Free PMC article.
-
Efficacy and safety of triptorelin 6-month formulation in patients with central precocious puberty.J Pediatr Endocrinol Metab. 2016 Nov 1;29(11):1241-1248. doi: 10.1515/jpem-2015-0376. J Pediatr Endocrinol Metab. 2016. PMID: 26887034 Clinical Trial.
-
Long-Acting Gonadotropin-Releasing Hormone Analogues for Central Precocious Puberty, Including 45-Mg 6-Month Subcutaneous Leuprolide Acetate: Use for Treatment and Treatment Monitoring.Horm Res Paediatr. 2025;98(3):258-265. doi: 10.1159/000539020. Epub 2024 Apr 23. Horm Res Paediatr. 2025. PMID: 38653206 Free PMC article. Review.
-
Triptorelin depot for the treatment of children 2 years and older with central precocious puberty.Expert Rev Clin Pharmacol. 2018 Jul;11(7):659-667. doi: 10.1080/17512433.2018.1494569. Epub 2018 Jul 26. Expert Rev Clin Pharmacol. 2018. PMID: 29957076 Review.
Cited by
-
A Phase 3, Open-Label, Single-Arm Trial of the Efficacy and Safety of Triptorelin 6-Month Formulation in Chinese Children with Central Precocious Puberty.Adv Ther. 2024 Dec;41(12):4537-4556. doi: 10.1007/s12325-024-02991-x. Epub 2024 Oct 16. Adv Ther. 2024. PMID: 39412628 Free PMC article. Clinical Trial.
-
Retrospective Analysis on the Impact of Triptorelin on Final Height of Girls with Precocious and Early Puberty: A Single-Center, Long-Term Study.Children (Basel). 2025 Jun 21;12(7):818. doi: 10.3390/children12070818. Children (Basel). 2025. PMID: 40723011 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

