IFN-γ production by brain-resident cells activates cerebral mRNA expression of a wide spectrum of molecules critical for both innate and T cell-mediated protective immunity to control reactivation of chronic infection with Toxoplasma gondii
- PMID: 36875520
- PMCID: PMC9975934
- DOI: 10.3389/fcimb.2023.1110508
IFN-γ production by brain-resident cells activates cerebral mRNA expression of a wide spectrum of molecules critical for both innate and T cell-mediated protective immunity to control reactivation of chronic infection with Toxoplasma gondii
Abstract
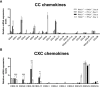
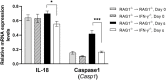
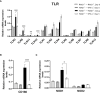
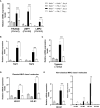
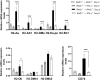
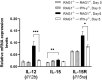
We previously demonstrated that brain-resident cells produce IFN-γ in response to reactivation of cerebral infection with Toxoplasma gondii. To obtain an overall landscape view of the effects of IFN-γ from brain-resident cells on the cerebral protective immunity, in the present study we employed NanoString nCounter assay and quantified mRNA levels for 734 genes in myeloid immunity in the brains of T and B cell-deficient, bone marrow chimeric mice with and without IFN-γ production by brain-resident cells in response to reactivation of cerebral T. gondii infection. Our study revealed that IFN-γ produced by brain-resident cells amplified mRNA expression for the molecules to activate the protective innate immunity including 1) chemokines for recruitment of microglia and macrophages (CCL8 and CXCL12) and 2) the molecules for activating those phagocytes (IL-18, TLRs, NOD1, and CD40) for killing tachyzoites. Importantly, IFN-γ produced by brain-resident cells also upregulated cerebral expression of molecules for facilitating the protective T cell immunity, which include the molecules for 1) recruiting effector T cells (CXCL9, CXCL10, and CXCL11), 2) antigen processing (PA28αβ, LMP2, and LMP7), transporting the processed peptides (TAP1 and TAP2), assembling the transported peptides to the MHC class I molecules (Tapasin), and the MHC class I (H2-K1 and H2-D1) and Ib molecules (H2-Q1, H-2Q2, and H2-M3) for presenting antigens to activate the recruited CD8+ T cells, 3) MHC class II molecules (H2-Aa, H2-Ab1, H2-Eb1, H2-Ea-ps, H2-DMa, H2-Ob, and CD74) to present antigens for CD4+ T cell activation, 4) co-stimulatory molecules (ICOSL) for T cell activation, and 5) cytokines (IL-12, IL-15, and IL-18) facilitating IFN-γ production by NK and T cells. Notably, the present study also revealed that IFN-γ production by brain-resident cells also upregulates cerebral expressions of mRNA for the downregulatory molecules (IL-10, STAT3, SOCS1, CD274 [PD-L1], IL-27, and CD36), which can prevent overly stimulated IFN-γ-mediated pro-inflammatory responses and tissue damages. Thus, the present study uncovered the previously unrecognized the capability of IFN-γ production by brain-resident cells to upregulate expressions of a wide spectrum of molecules for coordinating both innate and T cell-mediated protective immunity with a fine-tuning regulation system to effectively control cerebral infection with T. gondii.
Keywords: IFN-γ; Toxoplasma gondii; antigen presentation; brain-resident cells; chemokine; cytokine; protective immunity; regulatory molecule.
Copyright © 2023 Suzuki, Lutshumba, Chen, Abdelaziz, Sa and Ochiai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The immune system utilizes two distinct effector mechanisms of T cells depending on two different life cycle stages of a single pathogen, Toxoplasma gondii, to control its cerebral infection.Parasitol Int. 2020 Jun;76:102030. doi: 10.1016/j.parint.2019.102030. Epub 2019 Nov 25. Parasitol Int. 2020. PMID: 31778800 Free PMC article. Review.
-
[Rudolf-Virchow Prize 1998. Award lecture. Toxoplasmosis: a model infection for studying systemic and intracerebral immune reactions].Verh Dtsch Ges Pathol. 1998;82:9-22. Verh Dtsch Ges Pathol. 1998. PMID: 10095413 German.
-
Human MHC class I molecule, HLA-A2.1, mediates activation of CD8+ T cell IFN-γ production and the T cell-dependent protection against reactivation of cerebral Toxoplasma infection.Front Immunol. 2022 Oct 13;13:1005059. doi: 10.3389/fimmu.2022.1005059. eCollection 2022. Front Immunol. 2022. PMID: 36311799 Free PMC article.
-
Cutting Edge: IFN-γ Produced by Brain-Resident Cells Is Crucial To Control Cerebral Infection with Toxoplasma gondii.J Immunol. 2015 Aug 1;195(3):796-800. doi: 10.4049/jimmunol.1500814. Epub 2015 Jun 19. J Immunol. 2015. PMID: 26091720 Free PMC article.
-
Interferon-gamma- and perforin-mediated immune responses for resistance against Toxoplasma gondii in the brain.Expert Rev Mol Med. 2011 Oct 4;13:e31. doi: 10.1017/S1462399411002018. Expert Rev Mol Med. 2011. PMID: 22005272 Free PMC article. Review.
Cited by
-
Activatable Sulfur Dioxide Nanosonosensitizer Enables Precisely Controllable Sono-Gaseous Checkpoint Trimodal Therapy for Orthotopic Hepatocellular Carcinoma.Adv Sci (Weinh). 2025 Feb;12(5):e2409442. doi: 10.1002/advs.202409442. Epub 2024 Dec 16. Adv Sci (Weinh). 2025. PMID: 39679828 Free PMC article.
-
Toxoplasma gondii, endothelial cells and schizophrenia: is it just a barrier matter?Front Cell Infect Microbiol. 2025 Apr 10;15:1468936. doi: 10.3389/fcimb.2025.1468936. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40276385 Free PMC article. Review.
-
The Absence of CXCL10 Activity Does Not Affect the Capability of CD8+ T Cells to Migrate and Eliminate the Tissue Cysts of Toxoplasma gondii from the Brains of Chronically Infected Mice.Microorganisms. 2024 Oct 29;12(11):2172. doi: 10.3390/microorganisms12112172. Microorganisms. 2024. PMID: 39597561 Free PMC article.
-
Contrasting Disease Progression, Microglia Reactivity, Tolerance, and Resistance to Toxoplasma gondii Infection in Two Mouse Strains.Biomedicines. 2024 Jun 26;12(7):1420. doi: 10.3390/biomedicines12071420. Biomedicines. 2024. PMID: 39061995 Free PMC article.
-
Not Just PA28γ: What We Know About the Role of PA28αβ in Carcinogenesis.Biomolecules. 2025 Jun 16;15(6):880. doi: 10.3390/biom15060880. Biomolecules. 2025. PMID: 40563520 Free PMC article. Review.
References
-
- Andrade W. A., Souza Mdo C., Ramos-Martinez E., Nagpal K., Dutra M. S., Melo M. B., et al. . (2013). Combined action of nucleic acid-sensing toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe 13, 42–53. doi: 10.1016/j.chom.2012.12.003 - DOI - PMC - PubMed
-
- Andrade R. M., Wessendarp M., Gubbels M. J., Striepen B., Subauste C. S. (2006). CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J. Clin. Invest. 116, 2366–2377. doi: 10.1172/JCI28796 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

