Disease-associated oligodendrocyte signatures are spatiotemporally dysregulated in spinocerebellar ataxia type 3
- PMID: 36875652
- PMCID: PMC9975394
- DOI: 10.3389/fnins.2023.1118429
Disease-associated oligodendrocyte signatures are spatiotemporally dysregulated in spinocerebellar ataxia type 3
Abstract
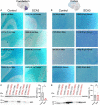
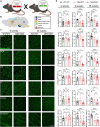
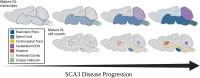
Spinocerebellar ataxia type 3 (SCA3) is a neurodegenerative disease caused by a CAG repeat expansion in the ATXN3 gene. Though the ATXN3 protein is expressed ubiquitously throughout the CNS, regional pathology in SCA3 patients is observed within select neuronal populations and more recently within oligodendrocyte-rich white matter tracts. We have previously recapitulated these white matter abnormalities in an overexpression mouse model of SCA3 and demonstrated that oligodendrocyte maturation impairments are one of the earliest and most progressive changes in SCA3 pathogenesis. Disease-associated oligodendrocyte signatures have recently emerged as significant contributors to several other neurodegenerative diseases, including Alzheimer's disease, Huntington's disease, and Parkinson's disease, but their role in regional vulnerability and disease progression remains unexplored. Here, we are the first to comparatively assess myelination in human tissue in a region-dependent manner. Translating these findings to SCA3 mouse models of disease, we confirmed endogenous expression of mutant Atxn3 leads to regional transcriptional dysregulation of oligodendrocyte maturation markers in Knock-In models of SCA3. We then investigated the spatiotemporal progression of mature oligodendrocyte transcriptional dysregulation in an overexpression SCA3 mouse model and how it relates to the onset of motor impairment. We further determined that regional reduction in mature oligodendrocyte cell counts in SCA3 mice over time parallels the onset and progression of brain atrophy in SCA3 patients. This work emphasizes the prospective contributions of disease-associated oligodendrocyte signatures to regional vulnerability and could inform timepoints and target regions imperative for biomarker assessment and therapeutic intervention in several neurodegenerative diseases.
Keywords: MJD; Machado-Joseph disease; SCA3; myelination; polyglutamine (polyQ) diseases.
Copyright © 2023 Schuster, DiFranco, Putka, Mato, Jarrah, Stec, Sundararajan and McLoughlin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Carvalho D. R., La Rocque-Ferreira A., Rizzo I. M., Imamura E. U., Speck-Martins C. E. (2008). Homozygosity enhances severity in spinocerebellar ataxia type 3. Pediatr. Neurol. 38 296–299. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

