Effects of percutaneously-implanted epidural stimulation on cardiovascular autonomic function and spasticity after complete spinal cord injury: A case report
- PMID: 36875669
- PMCID: PMC9978801
- DOI: 10.3389/fnins.2023.1112853
Effects of percutaneously-implanted epidural stimulation on cardiovascular autonomic function and spasticity after complete spinal cord injury: A case report
Abstract
Importance: There is a revived interest to explore spinal cord epidural stimulation (SCES) to improve physical function after spinal cord injury (SCI). This case report highlights the potential of eliciting multiple functional improvements with a single SCES configuration, a strategy which could improve clinical translation.
Objective: To determine whether SCES intended to facilitate walking also acutely yields benefits in cardiovascular autonomic regulation and spasticity.
Design: Case report from data collected at two timepoints 15 weeks apart from March to June 2022 as part of a larger clinical trial.
Setting: Research lab at Hunter Holmes McGuire VA Medical Center.
Participant: 27-year-old male, 7 years post a C8 motor complete spinal cord injury.
Intervention: A SCES configuration intended to enhance exoskeleton-assisted walking training applied for autonomic and spasticity management.
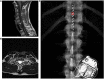
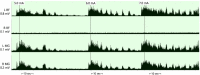
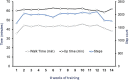
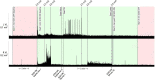
Main outcomes and measures: The primary outcome was cardiovascular autonomic response to a 45-degree head-up-tilt test. Systolic blood pressure (SBP), heart rate (HR), and absolute power of the low-frequency (LF) and high-frequency (HF) components of a heart-rate variability analysis were collected in supine and tilt with and without the presence of SCES. Right knee flexor and knee extensor spasticity was assessed via isokinetic dynamometry with and without SCES.
Results: At both assessments with SCES off, transitioning from supine to tilt decreased SBP (assessment one: 101.8 to 70 mmHg; assessment two: 98.9 to 66.4 mmHg). At assessment one, SCES on in supine (3 mA) increased SBP (average 117 mmHg); in tilt, 5 mA stabilized SBP near baseline values (average 111.5 mmHg). At assessment two, SCES on in supine (3 mA) increased SBP (average 140 mmHg in minute one); decreasing amplitude to 2 mA decreased SBP (average 119 mmHg in minute five). In tilt, 3 mA stabilized SBP near baseline values (average 93.2 mmHg). Torque-time integrals at the right knee were reduced at all angular velocities for knee flexors (range: -1.9 to -7.8%) and knee extensors (range: -1 to -11.4%).
Conclusions and relevance: These results demonstrate that SCES intended to facilitate walking may also enhance cardiovascular autonomic control and attenuate spasticity. Using one configuration to enhance multiple functions after SCI may accelerate clinical translation.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/, identifier NCT04782947.
Keywords: autonomic nervous system; exoskeleton; percutaneous epidural stimulation; rehabilitation; spasticity; spinal cord injury.
Copyright © 2023 Gorgey, Goldsmith, Alazzam and Trainer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Epidural Spinal Cord Stimulation for Spinal Cord Injury in Humans: A Systematic Review.J Clin Med. 2024 Feb 14;13(4):1090. doi: 10.3390/jcm13041090. J Clin Med. 2024. PMID: 38398403 Free PMC article. Review.
-
Epidural stimulation for cardiovascular function increases lower limb lean mass in individuals with chronic motor complete spinal cord injury.Exp Physiol. 2020 Oct;105(10):1684-1691. doi: 10.1113/EP088876. Epub 2020 Aug 15. Exp Physiol. 2020. PMID: 32749719
-
Spinal cord epidural stimulation for motor and autonomic function recovery after chronic spinal cord injury: A case series and technical note.Surg Neurol Int. 2023 Mar 17;14:87. doi: 10.25259/SNI_1074_2022. eCollection 2023. Surg Neurol Int. 2023. PMID: 37025529 Free PMC article.
-
Epidural Spinal Cord Stimulation of Lumbosacral Networks Modulates Arterial Blood Pressure in Individuals With Spinal Cord Injury-Induced Cardiovascular Deficits.Front Physiol. 2018 May 18;9:565. doi: 10.3389/fphys.2018.00565. eCollection 2018. Front Physiol. 2018. PMID: 29867586 Free PMC article.
-
Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis.Spinal Cord. 2012 Jul;50(7):484-92. doi: 10.1038/sc.2012.17. Epub 2012 Mar 6. Spinal Cord. 2012. PMID: 22391687 Review.
Cited by
-
Activity of spinal RORβ neurons is related to functional improvements following combination treatment after complete SCI.Proc Natl Acad Sci U S A. 2025 Apr 15;122(15):e2406333122. doi: 10.1073/pnas.2406333122. Epub 2025 Apr 8. Proc Natl Acad Sci U S A. 2025. PMID: 40198697
-
Epidural Spinal Cord Stimulation for Spinal Cord Injury in Humans: A Systematic Review.J Clin Med. 2024 Feb 14;13(4):1090. doi: 10.3390/jcm13041090. J Clin Med. 2024. PMID: 38398403 Free PMC article. Review.
-
Neuromodulation in Spinal Cord Injury Using Transcutaneous Spinal Stimulation-Mapping for a Blood Pressure Response: A Case Series.Neurotrauma Rep. 2024 Sep 20;5(1):845-856. doi: 10.1089/neur.2024.0066. eCollection 2024. Neurotrauma Rep. 2024. PMID: 39391052 Free PMC article.
-
Unintentionally intentional: unintended effects of spinal stimulation as a platform for multi-modal neurorehabilitation after spinal cord injury.Bioelectron Med. 2024 May 15;10(1):12. doi: 10.1186/s42234-024-00144-7. Bioelectron Med. 2024. PMID: 38745334 Free PMC article. Review.
-
Epidural Stimulation and Resistance Training (REST-SCI) for Overground Locomotion After Spinal Cord Injury: Randomized Clinical Trial Protocol.J Clin Med. 2025 Mar 8;14(6):1829. doi: 10.3390/jcm14061829. J Clin Med. 2025. PMID: 40142643 Free PMC article.
References
-
- Aslan S. C., Ditterline B. E., Park M. C., Angeli C. A., Rejc E., Chen Y., et al. (2018). Epidural spinal cord stimulation of lumbosacral networks modulates arterial blood pressure in individuals with spinal cord injury-induced cardiovascular deficits. Front. Physiol. 9:565. 10.3389/fphys.2018.00565 - DOI - PMC - PubMed
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

