Potential roles of the endoplasmic reticulum stress pathway in amyotrophic lateral sclerosis
- PMID: 36875699
- PMCID: PMC9974850
- DOI: 10.3389/fnagi.2023.1047897
Potential roles of the endoplasmic reticulum stress pathway in amyotrophic lateral sclerosis
Abstract
The endoplasmic reticulum (ER) is a major organelle involved in protein quality control and cellular homeostasis. ER stress results from structural and functional dysfunction of the organelle, along with the accumulation of misfolded proteins and changes in calcium homeostasis, it leads to ER stress response pathway such as unfolded protein response (UPR). Neurons are particularly sensitive to the accumulation of misfolded proteins. Thus, the ER stress is involved in neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, prion disease and motor neuron disease (MND). Recently, the complex involvement of ER stress pathways has been demonstrated in experimental models of amyotrophic lateral sclerosis (ALS)/MND using pharmacological and genetic manipulation of the unfolded protein response (UPR), an adaptive response to ER stress. Here, we aim to provide recent evidence demonstrating that the ER stress pathway is an essential pathological mechanism of ALS. In addition, we also provide therapeutic strategies that can help treat diseases by targeting the ER stress pathway.
Keywords: amyotrophic lateral sclerosis; endoplasmic reticulum stress; motor neuron disease; therapeutic target; unfolded protein response.
Copyright © 2023 Jeon, Kwon, Lee and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
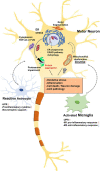
Figures

References
-
- Atkin J. D., Farg M. A., Turner B. J., Tomas D., Lysaght J. A., Nunan J., et al. . (2017). Withdrawal: induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J. Biol. Chem. 292:12007. doi: 10.1074/jbc.A117.603393, PMID: - DOI - PMC - PubMed
-
- Bellezza I., Grottelli S., Mierla A. L., Cacciatore I., Fornasari E., Roscini L., et al. . (2014). Neuroinflammation and endoplasmic reticulum stress are coregulated by cyclo(his-pro) to prevent LPS neurotoxicity. Int. J. Biochem. Cell Biol. 51, 159–169. doi: 10.1016/j.biocel.2014.03.023, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

