Redirecting T-cell Activity with Anti-BCMA/Anti-CD3 Bispecific Antibodies in Chronic Lymphocytic Leukemia and Other B-cell Lymphomas
- PMID: 36875718
- PMCID: PMC9981202
- DOI: 10.1158/2767-9764.CRC-22-0083
Redirecting T-cell Activity with Anti-BCMA/Anti-CD3 Bispecific Antibodies in Chronic Lymphocytic Leukemia and Other B-cell Lymphomas
Abstract
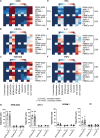
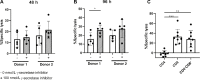
T-cell redirecting bispecific antibodies hold high promise for treatment of B-cell malignancies. B-cell maturation antigen (BCMA) exhibits high expression on normal and malignant mature B cells including plasma cells, which can be enhanced by inhibition of γ-secretase. BCMA is considered a validated target in multiple myeloma but whether mature B-cell lymphomas can be targeted by the BCMAxCD3 T-cell redirector teclistamab is currently unknown. BCMA expression on B-cell non-Hodgkin lymphoma and primary chronic lymphocytic leukemia (CLL) cells was assessed by flow cytometry and/or IHC. To assess teclistamab efficacy, cells were treated with teclistamab in presence of effector cells with/without γ-secretase inhibition. BCMA could be detected on all tested mature B-cell malignancy cell lines, while expression levels varied per tumor type. γ-secretase inhibition universally increased BCMA surface expression. These data were corroborated in primary samples from patients with Waldenstrom's macroglobulinemia, CLL, and diffuse large B-cell lymphoma. Functional studies with the B-cell lymphoma cell lines revealed teclistamab-mediated T-cell activation, proliferation, and cytotoxicity. This was independent of the level of BCMA expression, but generally lower in mature B-cell malignancies compared with multiple myeloma. Despite low BCMA levels, healthy donor T cells and CLL-derived T cells induced lysis of (autologous) CLL cells upon addition of teclistamab. These data show that BCMA is expressed on various B-cell malignancies and that lymphoma cell lines and primary CLL can be targeted using teclistamab. Further studies to understand the determinants of response to teclistamab are required to identify which other diseases might be suitable for teclistamab targeting.
Significance: Besides reported BCMA expression on multiple myeloma, we demonstrate BCMA can be detected and enhanced using γ-secretase inhibition on cell lines and primary material of various B-cell malignancies. Furthermore, using CLL we demonstrate that low BCMA-expressing tumors can be targeted efficiently using the BCMAxCD3 DuoBody teclistamab.
© 2022 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
N.W.C.J. van de Donk reports grants and other from Janssen Pharmaceuticals during the conduct of the study; grants and other from Amgen, Celgene/BMS, Novartis, Cellectis; other from Takeda, Roche, Bayer, Adaptive, and Servier outside the submitted work. H.C. Adams reports other from Janssen R&D outside the submitted work; in addition, H.C. Adams has a patent to PRD4087 issued. E. Eldering reports other from Janssen during the conduct of the study; grants from Janssen outside the submitted work. R. Verona reports other from Janssen R&D during the conduct of the study; other from Janssen R&D outside the submitted work; in addition, R. Verona has a patent to 63/194,470 pending. A.P. Kater reports grants from Janssen during the conduct of the study; grants and other from Abbvie, Genentech, AstraZeneca, BMS, and other from LAVA outside the submitted work; in addition, A.P. Kater has a patent to BCMA-bispecific for lymphoma pending. No other disclosures were reported.
Figures




References
-
- Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet 2017;390:298–310. - PubMed
-
- Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol 2019;94:1266–87. - PubMed
-
- Dreger P, Döhner H, Ritgen M, Böttcher S, Busch R, Dietrich S, et al. . Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood 2010;116:2438–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

