Chemodivergent C(sp3)-H and C(sp2)-H Cyanomethylation Using Engineered Carbene Transferases
- PMID: 36875868
- PMCID: PMC9983643
- DOI: 10.1038/s41929-022-00908-x
Chemodivergent C(sp3)-H and C(sp2)-H Cyanomethylation Using Engineered Carbene Transferases
Abstract
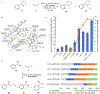
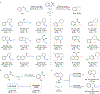
The ubiquity of C-H bonds presents an attractive opportunity to elaborate and build complexity in organic molecules. Methods for selective functionalization, however, often must differentiate among multiple chemically similar and, in some cases indistinguishable, C-H bonds. An advantage of enzymes is that they can be finely tuned using directed evolution to achieve control over divergent C-H functionalization pathways. Here, we demonstrate engineered enzymes that effect a new-to-nature C-H alkylation with unparalleled selectivity: two complementary carbene C-H transferases derived from a cytochrome P450 from Bacillus megaterium deliver an α-cyanocarbene into the α-amino C(sp3)-H bonds or the ortho-arene C(sp2)-H bonds of N-substituted arenes. These two transformations proceed via different mechanisms, yet only minimal changes to the protein scaffold (nine mutations, less than 2% of the sequence) were needed to adjust the enzyme's control over the site-selectivity of cyanomethylation. The X-ray crystal structure of the selective C(sp3)-H alkylase, P411-PFA, reveals an unprecedented helical disruption which alters the shape and electrostatics in the enzyme active site. Overall, this work demonstrates the advantages of enzymes as C-H functionalization catalysts for divergent molecular derivatization.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures
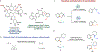
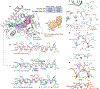
References
-
- Davies HML, Bois JD & Yu J-Q C–H functionalization in organic synthesis. Chem. Soc. Rev 40, 1855–1856 (2011). - PubMed
-
- Yamaguchi J, Yamaguchi AD & Itami K C–H bond functionalization: Emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed 51, 8960–9009 (2012). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
