Cardiovascular Toxicity of Proteasome Inhibitors: Underlying Mechanisms and Management Strategies: JACC: CardioOncology State-of-the-Art Review
- PMID: 36875897
- PMCID: PMC9982226
- DOI: 10.1016/j.jaccao.2022.12.005
Cardiovascular Toxicity of Proteasome Inhibitors: Underlying Mechanisms and Management Strategies: JACC: CardioOncology State-of-the-Art Review
Abstract
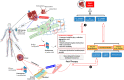
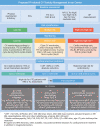
Proteasome inhibitors (PIs) are the backbone of combination treatments for patients with multiple myeloma and AL amyloidosis, while also indicated in Waldenström's macroglobulinemia and other malignancies. PIs act on proteasome peptidases, causing proteome instability due to accumulating aggregated, unfolded, and/or damaged polypeptides; sustained proteome instability then induces cell cycle arrest and/or apoptosis. Carfilzomib, an intravenous irreversible PI, exhibits a more severe cardiovascular toxicity profile as compared with the orally administered ixazomib or intravenous reversible PI such as bortezomib. Cardiovascular toxicity includes heart failure, hypertension, arrhythmias, and acute coronary syndromes. Because PIs are critical components of the treatment of hematological malignancies and amyloidosis, managing their cardiovascular toxicity involves identifying patients at risk, diagnosing toxicity early at the preclinical level, and offering cardioprotection if needed. Future research is required to elucidate underlying mechanisms, improve risk stratification, define the optimal management strategy, and develop new PIs with safe cardiovascular profiles.
Keywords: ACE, angiotensin-converting enzyme; ACS, acute coronary syndrome; AE, adverse event; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ASCT, autologous stem cell transplantation; BP, blood pressure; CVAE, cardiovascular adverse event; ESC, European Society of Cardiology; FMD, flow-mediated dilatation; GLS, global longitudinal strain; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; IHD, ischemic heart disease; IMiD, immunomodulatory drug; Kd, carfilzomib and dexamethasone; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; MM, multiple myeloma; NO, nitric oxide; NP, natriuretic peptide; OS, overall survival; PBMC, peripheral blood mononuclear cell; PFS, progression-free survival; PH, pulmonary hypertension; PI, proteasome inhibitor; PWV, pulse wave velocity; PrA, proteasome activity; RRMM, relapse or refractory multiple myeloma; SBP, systolic blood pressure; TMA, thrombotic microangiopathy; UPP, ubiquitin proteasome pathway; VTE, venous thromboembolism; Vd, bortezomib and dexamethasone; WM, Waldenström’s macroglobulinemia; bortezomib; cardiovascular toxicity; carfilzomib; eNOS, endothelial nitric oxide synthase; ixazomib; proteasome inhibition.
© 2023 The Authors.
Conflict of interest statement
Dr Janssen has been a consultant for Pfizer, Genesis Pharma, Amgen, and Takeda; has received research funding from Pfizer and Amgen; and has received honoraria and other paid expenses from Pfizer, Genesis Pharma, Amgen, and Takeda. Dr Dimopoulos has been a consultant, served on advisory boards, and has received personal fees/honoraria from BMS, Celgene, Takeda, Janssen, and Amgen; has received research funding from Takeda, Janssen, and Amgen; and has served on speakers bureaus for Celgene, Takeda, Janssen, and Amgen. Dr Stamatelopoulos has been a consultant for and received research funding and honoraria from Amgen. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


References
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. - PubMed
-
- Pohl C., Dikic I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science. 2019;366:818–822. - PubMed
-
- Zolk O., Schenke C., Sarikas A. The ubiquitin-proteasome system: focus on the heart. Cardiovasc Res. 2006;70:410–421. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

