Racing CARs to veterinary immuno-oncology
- PMID: 36876006
- PMCID: PMC9982037
- DOI: 10.3389/fvets.2023.1130182
Racing CARs to veterinary immuno-oncology
Abstract
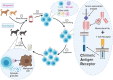
Chimeric antigen receptors (CARs) have demonstrated remarkable promise in human oncology over the past two decades, yet similar strategies in veterinary medicine are still in development. CARs are synthetically engineered proteins comprised of a specific antigen-binding single chain variable fragment (ScFv) fused to the signaling domain of a T cell receptor and co-receptors. Patient T cells engineered to express a CAR are directed to recognize and kill target cells, most commonly hematological malignancies. The U.S Food and Drug Administration (FDA) has approved multiple human CAR T therapies, but translation of these therapies into veterinary medicine faces many challenges. In this review, we discuss considerations for veterinary use including CAR design and cell carrier choice, and discuss the future promise of translating CAR therapy into veterinary oncology.
Keywords: applied immunology; cancer; cell therapy; immunotherapy; translational medicine.
Copyright © 2023 Cockey and Leifer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

