Reduction and prevention of agitation in persons with neurocognitive disorders: an international psychogeriatric association consensus algorithm
- PMID: 36876335
- PMCID: PMC10480345
- DOI: 10.1017/S104161022200103X
Reduction and prevention of agitation in persons with neurocognitive disorders: an international psychogeriatric association consensus algorithm
Abstract
Objectives: To develop an agitation reduction and prevention algorithm is intended to guide implementation of the definition of agitation developed by the International Psychogeriatric Association (IPA).
Design: Review of literature on treatment guidelines and recommended algorithms; algorithm development through reiterative integration of research information and expert opinion.
Setting: IPA Agitation Workgroup.
Participants: IPA panel of international experts on agitation.
Intervention: Integration of available information into a comprehensive algorithm.
Measurements: None.
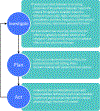
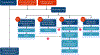
Results: The IPA Agitation Work Group recommends the Investigate, Plan, and Act (IPA) approach to agitation reduction and prevention. A thorough investigation of the behavior is followed by planning and acting with an emphasis on shared decision-making; the success of the plan is evaluated and adjusted as needed. The process is repeated until agitation is reduced to an acceptable level and prevention of recurrence is optimized. Psychosocial interventions are part of every plan and are continued throughout the process. Pharmacologic interventions are organized into panels of choices for nocturnal/circadian agitation; mild-moderate agitation or agitation with prominent mood features; moderate-severe agitation; and severe agitation with threatened harm to the patient or others. Therapeutic alternatives are presented for each panel. The occurrence of agitation in a variety of venues-home, nursing home, emergency department, hospice-and adjustments to the therapeutic approach are presented.
Conclusions: The IPA definition of agitation is operationalized into an agitation management algorithm that emphasizes the integration of psychosocial and pharmacologic interventions, reiterative assessment of response to treatment, adjustment of therapeutic approaches to reflect the clinical situation, and shared decision-making.
Keywords: International Psychogeriatric Association (IPA); agitation; algorithm; antipsychotics; emergency department; hospice; nocturnal agitation; pharmacotherapy; psychosocial intervention; shared decision-making.
Conflict of interest statement
Conflicts of Interest:
J. Cummings has provided consultation to Acadia, Alkahest, AlphaCognition, AriBio, Biogen, Cassava, Cortexyme, Diadem, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna, Lilly, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Otsuka, PRODEO, Prothena, ReMYND, Resverlogix, Roche, Signant Health, Suven, and United Neuroscience pharmaceutical, assessment, and investment companies. He is supported by NIGMS grant P20GM109025; NINDS grant U01NS093334; NIA grant R01AG053798; NIA grant P20AG068053; NIA grant P30AG072959; NIA grant R35AG71476; Alzheimer’s Disease Drug Discovery Foundation (ADDF); Ted and Maria Quirk Endowment; and the Joy Chambers-Grundy Endowment.
M. Sano receives grant support from NIH/NIA (P30AG066514, R24AG065163 R01AG051545) has provided consultation to Eisai, Avenir, vTv, Biogen, BioXcel, F.Hoffman LaRoche Merck NovoNordisk and Novartis; Is the chair of the DSMB for a Phase II Trial to Evaluate Safety and Efficacy of GM-CSF/Sargramostim in Alzheimer’s Disease (SESAD) (sponsor: University of Colorado); Alzheimer Association Member of Medical and Scientific Advisory Group. S. Auer was supported by grants of the Austrian Science Fund (FWF; I 2361-B27), the Federal State of Lower Austria (K3-F-907/001–2020) and has no financial relationships to disclose.
S. Bergh has no disclosures.
C. Fischer receives funding from Brain Canada, the Weston Foundation, NIH, Vielight Inc and Hoffman La Roche and has no financial relationships to disclose.
D. Gerritsen has no financial relationships to disclose.
G. Grossberg is a consultant to Acadia; Avanir; Axsome; BipXcel; Biogen; Genentech; Karuna; Lundbeck; Otsuka; Roche; Takeda and he receives research Support from HRSA; NIA; Functional Neuromodulation; He is also a member of Safety Monitoring Committees for Anavex; EryDel; Intra-Cellular; Merck; Newron;
Z. Ismail has received honoraria from Lundbeck/Otsuka. His institution has received fees from Acadia, Biogen, and Roche;
K. L. Lanctôt is supported by the Alzheimer’s Drug Discovery Foundation (ADDF) and the Bernick Chair in Geriatric Psychopharmacology, has provided consultation to BioXcel Therapeutics, Bright Minds, Cerevel Therapeutics, Eisai Co. Ltd., ICG Pharma, Jazz Pharmaceuticals, Otsuka, Kondor Pharma, H Lundbeck A/S, Merck Sharp Dohme, Novo Nordisk, Praxis Therapeutics;
M. I. Lapid, MD was supported in part by grants R01AG061100 from the National Institute on Aging and has no financial relationships to disclose.
J. Mintzer is a consultant for: Acadia Pharmaceuticals, Genentech, Ironshore Pharmaceuticals, Praxis Bioresearch, and Sygnature Discovery and a Steering Committee Member/Association Member for the Alzheimer’s Clinical Trials Consortium, the International Psychogeriatric Association, and the Technology Accelerator Company and a majority partner for Recruitment Partners and a Stockholder and VP of Clinical Affairs for NeuroQuest;
R. Palm has no disclosures.
P. B. Rosenberg, M.D. - was supported in part by grants R01AG054771 and R01AG050515 from the National Institute on Aging and has no financial relationships to disclose.
K. Zhong has no disclosures.
M. Splaine has no disclosures.
C. Zhu has no disclosures.
Figures
References
-
- Airagnes G, Pelissolo A, Lavallee M, Flament M and Limosin F (2016). Benzodiazepine misuse in the elderly: risk factors, consequences, and management. Curr Psychiatry Rep, 18, 89. - PubMed
-
- Anderson JG, Hundt E and Rose KM (2019). Nonpharmacological strategies used by family caregivers of persons with Alzheimer’s disease and related dementias as presented in blogs. J Gerontol Nurs, 45, 25–35. - PubMed
-
- Ballard CG, Coate B, Abler V, Stankovic S and Foff E (2020). Evaluation of the efficacy of pimavanserin in the treatment of agitation and aggression in patients with Alzheimer’s disease psychosis: A post hoc analysis. Int J Geriatr Psychiatry, 35, 1402–1408. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 AG066507/AG/NIA NIH HHS/United States
- P20 AG068053/AG/NIA NIH HHS/United States
- R01 AG050515/AG/NIA NIH HHS/United States
- U01 NS093334/NS/NINDS NIH HHS/United States
- P30 AG072980/AG/NIA NIH HHS/United States
- R01 AG051545/AG/NIA NIH HHS/United States
- P20 GM109025/GM/NIGMS NIH HHS/United States
- R01 AG053798/AG/NIA NIH HHS/United States
- R01 AG054771/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- R01 AG061100/AG/NIA NIH HHS/United States
- R35 AG071476/AG/NIA NIH HHS/United States
- P30 AG066514/AG/NIA NIH HHS/United States
- P30 AG072959/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources

