Transferred mitochondria accumulate reactive oxygen species, promoting proliferation
- PMID: 36876914
- PMCID: PMC10042539
- DOI: 10.7554/eLife.85494
Transferred mitochondria accumulate reactive oxygen species, promoting proliferation
Abstract
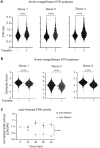
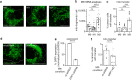
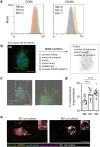
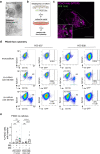
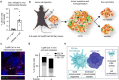
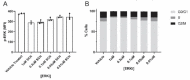
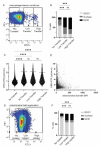
Recent studies reveal that lateral mitochondrial transfer, the movement of mitochondria from one cell to another, can affect cellular and tissue homeostasis. Most of what we know about mitochondrial transfer stems from bulk cell studies and have led to the paradigm that functional transferred mitochondria restore bioenergetics and revitalize cellular functions to recipient cells with damaged or non-functional mitochondrial networks. However, we show that mitochondrial transfer also occurs between cells with functioning endogenous mitochondrial networks, but the mechanisms underlying how transferred mitochondria can promote such sustained behavioral reprogramming remain unclear. We report that unexpectedly, transferred macrophage mitochondria are dysfunctional and accumulate reactive oxygen species in recipient cancer cells. We further discovered that reactive oxygen species accumulation activates ERK signaling, promoting cancer cell proliferation. Pro-tumorigenic macrophages exhibit fragmented mitochondrial networks, leading to higher rates of mitochondrial transfer to cancer cells. Finally, we observe that macrophage mitochondrial transfer promotes tumor cell proliferation in vivo. Collectively these results indicate that transferred macrophage mitochondria activate downstream signaling pathways in a ROS-dependent manner in cancer cells, and provide a model of how sustained behavioral reprogramming can be mediated by a relatively small amount of transferred mitochondria in vitro and in vivo.
Keywords: cancer; cancer biology; cell biology; cell proliferation; cell-cell communication; human; macrophages; mitochondrial transfer; mouse; reactive oxygen species.
© 2023, Kidwell, Casalini et al.
Conflict of interest statement
CK, JC, SP, SS, DG, DB, DW, GO, JL, JJ, JR, AW, TZ, MR No competing interests declared
Figures









Update of
References
-
- Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. The EMBO Journal. 2014;33:994–1010. doi: 10.1002/embj.201386030. - DOI - PMC - PubMed
-
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: A graded response to membrane stimulation. Journal of Immunology. 1983;130:1910–1917. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- U54 CA224076/CA/NCI NIH HHS/United States
- F31 CA250317/CA/NCI NIH HHS/United States
- TL1 TR002549/TR/NCATS NIH HHS/United States
- F32 CA159663/CA/NCI NIH HHS/United States
- K99 CA248611/CA/NCI NIH HHS/United States
- R37 CA247994/CA/NCI NIH HHS/United States
- R00 CA190836/CA/NCI NIH HHS/United States
- R37CA247994/NH/NIH HHS/United States
- K99 CA248611/NH/NIH HHS/United States
- R35 GM131854/GM/NIGMS NIH HHS/United States
- F31CA250317/NH/NIH HHS/United States
- TL1 TR002549/NH/NIH HHS/United States
- U54CA224076/NH/NIH HHS/United States
- R00CA190836/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

