MitoStores: a place for precursors to ride out the storm
- PMID: 36876922
- PMCID: PMC10068307
- DOI: 10.15252/embj.2023113576
MitoStores: a place for precursors to ride out the storm
Abstract
The fate of unimported mitochondrial precursors has been increasingly studied in recent years, mostly focusing on protein degradation. In this issue of the EMBO journal, Krämer et al discovered MitoStores, a new protective mechanism that temporarily stores mitochondrial proteins in cytosolic deposits.
© 2023 The Authors.
Figures
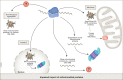
Comment on
-
MitoStores: chaperone-controlled protein granules store mitochondrial precursors in the cytosol.EMBO J. 2023 Apr 3;42(7):e112309. doi: 10.15252/embj.2022112309. Epub 2023 Jan 27. EMBO J. 2023. PMID: 36704946 Free PMC article.
References
-
- Boos F, Krämer L, Groh C, Jung F, Haberkant P, Stein F, Wollweber F, Gackstatter A, Zöller E, van der Laan M et al (2019) Mitochondrial protein‐induced stress triggers a global adaptive transcriptional programme. Nat Cell Biol 21: 442–451 - PubMed
-
- Mårtensson CU, Priesnitz C, Song J, Ellenrieder L, Doan KN, Boos F, Floerchinger A, Zufall N, Oeljeklaus S, Warscheid B et al (2019) Mitochondrial protein translocation‐associated degradation. Nature 569: 679–683 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

