Stringent Response-Mediated Control of GTP Homeostasis Is Required for Long-Term Viability of Staphylococcus aureus
- PMID: 36877013
- PMCID: PMC10101089
- DOI: 10.1128/spectrum.00447-23
Stringent Response-Mediated Control of GTP Homeostasis Is Required for Long-Term Viability of Staphylococcus aureus
Abstract
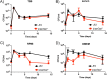
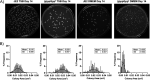
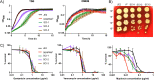
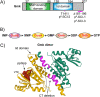
Staphylococcus aureus is an opportunistic bacterial pathogen that often results in difficult-to-treat infections. One mechanism used by S. aureus to enhance survival during infection is the stringent response. This is a stress survival pathway that utilizes the nucleotides (p)ppGpp to reallocate bacterial resources, shutting down growth until conditions improve. Small colony variants (SCVs) of S. aureus are frequently associated with chronic infections, and this phenotype has previously been linked to a hyperactive stringent response. Here, we examine the role of (p)ppGpp in the long-term survival of S. aureus under nutrient-restricted conditions. When starved, a (p)ppGpp-null S. aureus mutant strain ((p)ppGpp0) initially had decreased viability. However, after 3 days we observed the presence and dominance of a population of small colonies. Similar to SCVs, these small colony isolates (p0-SCIs) had reduced growth but remained hemolytic and sensitive to gentamicin, phenotypes that have been tied to SCVs previously. Genomic analysis of the p0-SCIs revealed mutations arising within gmk, encoding an enzyme in the GTP synthesis pathway. We show that a (p)ppGpp0 strain has elevated levels of GTP, and that the mutations in the p0-SCIs all lower Gmk enzyme activity and consequently cellular GTP levels. We further show that in the absence of (p)ppGpp, cell viability can be rescued using the GuaA inhibitor decoyinine, which artificially lowers the intracellular GTP concentration. Our study highlights the role of (p)ppGpp in GTP homeostasis and underscores the importance of nucleotide signaling for long-term survival of S. aureus in nutrient-limiting conditions, such as those encountered during infections. IMPORTANCE Staphylococcus aureus is a human pathogen that upon invasion of a host encounters stresses, such as nutritional restriction. The bacteria respond by switching on a signaling cascade controlled by the nucleotides (p)ppGpp. These nucleotides function to shut down bacterial growth until conditions improve. Therefore, (p)ppGpp are important for bacterial survival and have been implicated in promoting chronic infections. Here, we investigate the importance of (p)ppGpp for long-term survival of bacteria in nutrient-limiting conditions similar to those in a human host. We discovered that in the absence of (p)ppGpp, bacterial viability decreases due to dysregulation of GTP homeostasis. However, the (p)ppGpp-null bacteria were able to compensate by introducing mutations in the GTP synthesis pathway that led to a reduction in GTP build-up and a rescue of viability. This study therefore highlights the importance of (p)ppGpp for the regulation of GTP levels and for long-term survival of S. aureus in restricted environments.
Keywords: (p)ppGpp; GTP; Staphylococcus aureus; stringent response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
(p)ppGpp-mediated GTP homeostasis ensures survival and antibiotic tolerance of Staphylococcus aureus.Commun Biol. 2025 Mar 28;8(1):508. doi: 10.1038/s42003-025-07910-6. Commun Biol. 2025. PMID: 40155724 Free PMC article.
-
The Stringent Response Inhibits 70S Ribosome Formation in Staphylococcus aureus by Impeding GTPase-Ribosome Interactions.mBio. 2021 Dec 21;12(6):e0267921. doi: 10.1128/mBio.02679-21. Epub 2021 Nov 9. mBio. 2021. PMID: 34749534 Free PMC article.
-
(p)ppGpp/GTP and Malonyl-CoA Modulate Staphylococcus aureus Adaptation to FASII Antibiotics and Provide a Basis for Synergistic Bi-Therapy.mBio. 2021 Feb 2;12(1):e03193-20. doi: 10.1128/mBio.03193-20. mBio. 2021. PMID: 33531402 Free PMC article.
-
Staphylococcus aureus Small Colony Variants (SCVs): a road map for the metabolic pathways involved in persistent infections.Front Cell Infect Microbiol. 2014 Jul 28;4:99. doi: 10.3389/fcimb.2014.00099. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25120957 Free PMC article. Review.
-
The stringent response and Mycobacterium tuberculosis pathogenesis.Pathog Dis. 2018 Jul 1;76(5):fty054. doi: 10.1093/femspd/fty054. Pathog Dis. 2018. PMID: 29947752 Free PMC article. Review.
Cited by
-
(p)ppGpp-mediated GTP homeostasis ensures survival and antibiotic tolerance of Staphylococcus aureus.Commun Biol. 2025 Mar 28;8(1):508. doi: 10.1038/s42003-025-07910-6. Commun Biol. 2025. PMID: 40155724 Free PMC article.
-
Tale of Twin Bifunctional Second Messenger (p)ppGpp Synthetases and Their Function in Mycobacteria.ACS Omega. 2023 Aug 25;8(36):32258-32270. doi: 10.1021/acsomega.3c03557. eCollection 2023 Sep 12. ACS Omega. 2023. PMID: 37720788 Free PMC article. Review.
References
-
- Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, Schrenzel J, Lalk M, Wolz C. 2012. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog 8:e1003016. doi: 10.1371/journal.ppat.1003016. - DOI - PMC - PubMed
-
- Gao W, Chua K, Davies JK, Newton HJ, Seemann T, Harrison PF, Holmes NE, Rhee HW, Hong JI, Hartland EL, Stinear TP, Howden BP. 2010. Two novel point mutations in clinical Staphylococcus aureus reduce linezolid susceptibility and switch on the stringent response to promote persistent infection. PLoS Pathog 6:e1000944. doi: 10.1371/journal.ppat.1000944. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

