Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial
- PMID: 36877118
- PMCID: PMC10158608
- DOI: 10.1161/CIRCULATIONAHA.123.063988
Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial
Abstract
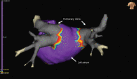
Background: Pulsed field ablation uses electrical pulses to cause nonthermal irreversible electroporation and induce cardiac cell death. Pulsed field ablation may have effectiveness comparable to traditional catheter ablation while preventing thermally mediated complications.
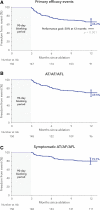
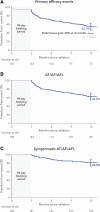
Methods: The PULSED AF pivotal study (Pulsed Field Ablation to Irreversibly Electroporate Tissue and Treat AF) was a prospective, global, multicenter, nonrandomized, paired single-arm study in which patients with paroxysmal (n=150) or persistent (n=150) symptomatic atrial fibrillation (AF) refractory to class I or III antiarrhythmic drugs were treated with pulsed field ablation. All patients were monitored for 1 year using weekly and symptomatic transtelephonic monitoring; 3-, 6-, and 12-month ECGs; and 6- and 12-month 24-hour Holter monitoring. The primary effectiveness end point was freedom from a composite of acute procedural failure, arrhythmia recurrence, or antiarrhythmic escalation through 12 months, excluding a 3-month blanking period to allow recovery from the procedure. The primary safety end point was freedom from a composite of serious procedure- and device-related adverse events. Kaplan-Meier methods were used to evaluate the primary end points.
Results: Pulsed field ablation was shown to be effective at 1 year in 66.2% (95% CI, 57.9 to 73.2) of patients with paroxysmal AF and 55.1% (95% CI, 46.7 to 62.7) of patients with persistent AF. The primary safety end point occurred in 1 patient (0.7%; 95% CI, 0.1 to 4.6) in both the paroxysmal and persistent AF cohorts.
Conclusions: PULSED AF demonstrated a low rate of primary safety adverse events (0.7%) and provided effectiveness consistent with established ablation technologies using a novel irreversible electroporation energy to treat patients with AF.
Registration: URL: https://www.
Clinicaltrials: gov; Unique identifier: NCT04198701.
Keywords: atrial fibrillation; catheter ablation; clinical trial; electroporation; pulsed field ablation.
Conflict of interest statement
Figures

Comment in
-
Non-thermal ablation of AF is safe and effective.Nat Rev Cardiol. 2023 May;20(5):286. doi: 10.1038/s41569-023-00865-3. Nat Rev Cardiol. 2023. PMID: 36944788 No abstract available.
-
Pulsed Field Ablation: Is It Better Than Conventional Thermal Ablation for Treatment of Atrial Fibrillation?Circulation. 2023 May 9;147(19):1433-1435. doi: 10.1161/CIRCULATIONAHA.123.064329. Epub 2023 May 8. Circulation. 2023. PMID: 37155587 No abstract available.
-
Letter by Krisai et al Regarding Article, "Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial".Circulation. 2023 Oct 10;148(15):1182. doi: 10.1161/CIRCULATIONAHA.123.065076. Epub 2023 Oct 9. Circulation. 2023. PMID: 37812654 No abstract available.
References
-
- Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. . 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160. doi: 10.1093/europace/eux274 - PMC - PubMed
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. ; ESC Scientific Document Group. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 - PubMed
-
- Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, et al. . Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116 - PubMed
-
- Yarmush ML, Golberg A, Sersa G, Kotnik T, Miklavcic D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

