Impact of active placebo controls on estimated drug effects in randomised trials: a systematic review of trials with both active placebo and standard placebo
- PMID: 36877132
- PMCID: PMC9989326
- DOI: 10.1002/14651858.MR000055.pub2
Impact of active placebo controls on estimated drug effects in randomised trials: a systematic review of trials with both active placebo and standard placebo
Abstract
Background: An estimated 60% of pharmacological randomised trials use placebo control interventions to blind (i.e. mask) participants. However, standard placebos do not control for perceptible non-therapeutic effects (i.e. side effects) of the experimental drug, which may unblind participants. Trials rarely use active placebo controls, which contain pharmacological compounds designed to mimic the non-therapeutic experimental drug effects in order to reduce the risk of unblinding. A relevant improvement in the estimated effects of active placebo compared with standard placebo would imply that trials with standard placebo may overestimate experimental drug effects.
Objectives: We aimed to estimate the difference in drug effects when an experimental drug is compared with an active placebo versus a standard placebo control intervention, and to explore causes for heterogeneity. In the context of a randomised trial, this difference in drug effects can be estimated by directly comparing the effect difference between the active placebo and standard placebo intervention.
Search methods: We searched PubMed, CENTRAL, Embase, two other databases, and two trial registries up to October 2020. We also searched reference lists and citations and contacted trial authors.
Selection criteria: We included randomised trials that compared an active placebo versus a standard placebo intervention. We considered trials both with and without a matching experimental drug arm.
Data collection and analysis: We extracted data, assessed risk of bias, scored active placebos for adequacy and risk of unintended therapeutic effect, and categorised active placebos as unpleasant, neutral, or pleasant. We requested individual participant data from the authors of four cross-over trials published after 1990 and one unpublished trial registered after 1990. Our primary inverse-variance, random-effects meta-analysis used standardised mean differences (SMDs) of active versus standard placebo for participant-reported outcomes at earliest post-treatment assessment. A negative SMD favoured the active placebo. We stratified analyses by trial type (clinical or preclinical) and supplemented with sensitivity and subgroup analyses and meta-regression. In secondary analyses, we investigated observer-reported outcomes, harms, attrition, and co-intervention outcomes.
Main results: We included 21 trials (1462 participants). We obtained individual participant data from four trials. Our primary analysis of participant-reported outcomes at earliest post-treatment assessment resulted in a pooled SMD of -0.08 (95% confidence interval (CI) -0.20 to 0.04; I2 = 31%; 14 trials), with no clear difference between clinical and preclinical trials. Individual participant data contributed 43% of the weight of this analysis. Two of seven sensitivity analyses found more pronounced and statistically significant differences; for example, in the five trials with low overall risk of bias, the pooled SMD was -0.24 (95% CI -0.34 to -0.13). The pooled SMD of observer-reported outcomes was similar to the primary analysis. The pooled odds ratio (OR) for harms was 3.08 (95% CI 1.56 to 6.07), and for attrition, 1.22 (95% CI 0.74 to 2.03). Co-intervention data were limited. Meta-regression found no statistically significant association with adequacy of the active placebo or risk of unintended therapeutic effect.
Authors' conclusions: We did not find a statistically significant difference between active and standard placebo control interventions in our primary analysis, but the result was imprecise and the CI compatible with a difference ranging from important to irrelevant. Furthermore, the result was not robust, because two sensitivity analyses produced a more pronounced and statistically significant difference. We suggest that trialists and users of information from trials carefully consider the type of placebo control intervention in trials with high risk of unblinding, such as those with pronounced non-therapeutic effects and participant-reported outcomes.
Trial registration: ClinicalTrials.gov NCT02194088 NCT02629146 NCT02283281.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MRH has received grants from Pfizer, paid to his employer, outside the submitted work, has received speaking fees from Novartis outside the submitted work, and owns stocks in Novo Nordisk. During the editorial process, MRH changed employment from Odense University Hospital and University of Southern Denmark to Novo Nordisk on 1 February 2022; this was after submission of the first review draft and response to peer review comments, but before the copy editing. All three affiliations are therefore listed.
JP, EB and CPW are co‐authors of three of the included studies and were not involved in the study inclusion, data extraction, and risk of bias assessment of any studies.
The remaining authors (DRTL, CHN, ADF, ASP, AH) declare no financial or non‐financial conflicts of interest.
Figures
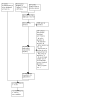


































Update of
References
References to studies included in this review
Adelson 1962 {published data only}
Berna 2017 {published data only}
Bjørkedal 2011 {published and unpublished data}
-
- Bjørkedal E, Nermo T, Aslaksen PM, Flaten MA. The effect of an active placebo on pain-induced event related potentials in a balanced placebo design. Psychophysiology 2009;46:S127. [DOI: 10.1111/j.1469-8986.2009.00920.x] - DOI
-
- Bjørkedal E. Active placebo – the relation of treatment expectancies to active analgesic treatments. The Arctic University of Norway (UiT) 2016. [hdl.handle.net/10037/10017]
-
- Flaten M, Aslaksen P, Bjørkedal E. Event-related potentials to painful stimulation after caffeine and placebo in a balanced placebo design. Journal of Pain 2010;11(4):S56. [DOI: 10.1016/j.jpain.2010.01.233] - DOI
Casey 1960 {published data only}
-
- Casey JF, Bennett IF, Lindley CJ, Hollister LE, Gordon MH, Springer NN. Drug therapy in schizophrenia. A controlled study of the relative effectiveness of chlorpromazine, promazine, phenobarbital, and placebo. A.M.A. Archives of General Psychiatry 1960;2(2):210-20. [DOI: 10.1001/archpsyc.1960.03590080086012] [PMID: ] - DOI - PubMed
-
- Lindley CJ, editor(s). Transactions of the Second (1957) Research Conference on Chemotherapy in Psychiatry. Vol. 2. Washington, D.C.: Veterans Administration, 1958.
Dellemijn 1997 {published data only}
Eriksson 2019 (sensitivity) {published data only}
-
- Eriksson L, Asadi J. Intraoral injections of active/non-active local anesthetic agents – normal response and response in relation to perceived numbness. [Intraoral injektion av aktiv och icke-aktiv lokalanestetika – normalt gensvar och gensvar i relation till upplevd bedövningskänsla]. Malmö universitet/Odontologiska fakulteten 2019. [DIVA, ID: diva2:1479798]
Fangman 1963 {published data only}
-
- Fangman JJ. The effects of morphine on two forms of experimental pain. ETD Collection for Fordham University 1963:1-81. [https://research.library.fordham.edu/dissertations/AAI6305590]
Gracely 1987 (sensitivity) {published data only}
Greenbaum 1987 {published data only}
Kurland 1961 {published data only}
-
- Kurland AA, Hanlon TE, Tatom MH, Ota KY, Simopoulos AM. The comparative effectiveness of six phenothiazine compounds, phenobarbital, and inert placebo in treatment of acutely ill patients: global measures of severity of illness. Journal of Nervous and Mental Disease 1961;133(1):1-18. [DOI: 10.1097/00005053-196107000-00001] [PMID: ] - DOI - PubMed
-
- Kurland AA, Hanlon TE, Tatom MH, Simopoulos AM. Comparative studies of the phenothiazine tranquilizers: methodological and logistical considerations. Journal of Nervous and Mental Disease 1961;132:61-74. [PMID: ] - PubMed
-
- Kurland AA, Michaux MH, Hanlon TE, Ota KY, Simopoulos AM. The comparative effectiveness of six phenothiazine compounds, phenobarbital and inert placebo in the treatment of acutely ill patients: personality dimensions. Journal of Nervous and Mental Disease 1962;134(1):48-60. [PMID: ] - PubMed
-
- Kurland AA, Sutherland GF. The phenothiazine tranquilizers – their neurological complications and significance. Psychosomatics 1960;1(4):192-4. [DOI: 10.1016/S0033-3182(60)72984-0] - DOI
Letemendia 1959 {published data only}
-
- Letemendia F, Harris AD. The right kind of control. Lancet 1959;273(7076):785-6. [DOI: 10.1016/S0140-6736(59)91863-X] - DOI
Lipman 1966 {published data only}
Malmstrom 2006 {published data only}
Max 1988 (sensitivity) {published data only}
McKinley 2016 {published data only}
-
- McKinley M. The influence of side effects on the nocebo and placebo response. University of Auckland 2016. [hdl.handle.net/2292/34346]
Parkes 2015 {published data only}
-
- Parkes B. The effect of medication price on placebo and nocebo responding. University of Auckland 2015. [hdl.handle.net/2292/29075]
Prosenz 2017 {published and unpublished data}
Rief 2012 {published data only}
-
- Rief W. The relevance of placebo effects in clinical trials. Journal of Psychosomatic Research 2012;72(6):498. [DOI: 10.1016/j.jpsychores.2012.03.004] - DOI
Shader 1964 {published data only}
Solomon 1960 {published data only}
-
- Gordan GS, Vaughan C. Prevention and treatment of postmenopausal osteoporosis. In: Pasetto N, Paoletti R, Ambrus JL, editors(s). The menopause and postmenopause: the proceedings of an international symposium held in Rome, June 1979. Dordrecht: Springer Netherlands, 1980:179-93. [DOI: 10.1007/978-94-011-7230-1_18] - DOI
-
- Solomon GF, Dickerson WJ, Eisenberg E. Psychologic and osteometabolic responses to sex hormones in elderly osteoporotic women. Geriatrics 1960;15:46-60. [PMID: ] - PubMed
Wang 2008a {published data only}
-
- Wang H, Bolognese J, Calder N, Baxendale J, Kehler A, Cummings C, et al. Effect of morphine and pregabalin compared with diphenhydramine hydrochloride and placebo on hyperalgesia and allodynia induced by intradermal capsaicin in healthy male subjects. Journal of Pain 2008;9(12):1088-95. [DOI: 10.1016/j.jpain.2008.05.013] [PMID: ] - DOI - PubMed
Werner 2019a {published and unpublished data}
-
- Werner C, Faasse K, Sharpe L, Colagiuri B. The development and evaluation of a new model of an active placebo. In: 2nd official Society for Interdisciplinary Placebo Studies (SIPS) conference. Leiden, 2019.
-
- Werner C. The development and evaluation of a new experimental model of an active placebo to investigate how the experience of side effects influences the placebo effects. University of Sydney 2022. [https://hdl.handle.net/2123/27394]
Werner 2019b {unpublished data only}
-
- Unpublished data. Trial data obtained from trial author.
-
- Werner C. The development and evaluation of a new experimental model of an active placebo to investigate how the experience of side effects influences the placebo effects. The University of Sydney 2022. [https://hdl.handle.net/2123/27394]
Woodrow 1988 {published data only}
References to studies excluded from this review
Abse 1956 {published data only}
Eriksson 2019 {published data only}
-
- Eriksson L, Asadi J. Intraoral injections of active/non-active local anesthetic agents – normal response and response in relation to perceived numbness. [Intraoral injektion av aktiv och icke-aktiv lokalanestetika – normalt gensvar och gensvar i relation till upplevd bedövningskänsla]. Malmö universitet/Odontologiska fakulteten 2019. [DIVA, ID: diva2:1479798]
Gracely 1987 {published data only}
Ibera 2018 {published data only}
-
- Ibera C, Shalom B, Saifi F, Shruder J, Davidson E. Effects of cannabis extract premedication on anesthetic depth. Harefuah 2018;157(3):162-6. [PMID: ] - PubMed
Max 1988 {published data only}
Yamagata 1973 {published data only}
-
- Yamagata S, Ishimori A, Sato H, Okabe H, Ishihara K. Clinical evaluation of pharmacotherapy for peptic ulcer with antipepsin agents by double blind technique – multicenter clinical study. Tohoku Journal of Experimental Medicine 1973;110(4):377-404. [DOI: 10.1620/tjem.110.377] [PMID: ] - DOI - PubMed
Yuge 1973 {published data only}
-
- Yuge J, Tsukada O, Nito H, Saito T, Fukuda S, Nishimura Y, et al. A clinical trial on analgesic effect of 1,3,5-trihydroxybenzene (Dilospan inj.) – Simple double blind test by the cases of urolithiasis [1,3,5-trihydroxybenzene(Dilospan注)の鎮痛効果に関する臨床実験 –尿路結石症を対象とした単純二重盲検実験]. Hinyokika Kiyo 1973;19(1):119-25. [hdl.handle.net/2433/121473]
References to studies awaiting assessment
Additional references
Aan Het Rot 2012
Bello 2016
-
- Bello S, Wei M, Hilden J, Hróbjartsson A. The matching quality of experimental and control interventions in blinded pharmacological randomised clinical trials: a methodological systematic review. BMC Medical Research Methodology 2016;16:18. [DOI: 10.1186/s12874-016-0111-9] [PMID: ] - DOI - PMC - PubMed
Boutron 2006
Chan 2005
Chinn 2000
Cohen 1988
-
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Routledge, 1988.
Colagiuri 2010
Dechartres 2016
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Gaudiano 2005
Greenberg 1994
Gøtzsche 2007
Higgins 2021a
-
- Higgins JP, Savovic J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021b
-
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.1.
Hopewell 2010
Hróbjartsson 2011
Hróbjartsson 2014
-
- Hróbjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. International Journal of Epidemiology 2014;43(4):1272-83. [DOI: 10.1093/ije/dyu115] [PMID: ] - DOI - PMC - PubMed
Jakobsen 2017
-
- Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller K, et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC psychiatry 2017;17(1):58. [DOI: 10.1186/s12888-016-1173-2] [PMID: ] - DOI - PMC - PubMed
Jensen 2017
Johnson 1940
-
- Johnson NL, Welch BL. Applications of the non-central t-distribution. Biometrika 1940;31(3-4):362-89. [DOI: 10.1093/biomet/31.3-4.362] - DOI
Laursen 2020
-
- Laursen DR, Hansen C, Paludan-Müller AS, Hróbjartsson A. Active placebo versus standard placebo control interventions in pharmacological randomised trials. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: MR000055. [DOI: 10.1002/14651858.MR000055] - DOI
Madeyski 2018
-
- Madeyski L, Kitchenham B. Effect sizes and their variance for AB/BA crossover design studies. Empirical Software Engineering 2018;23(4):1982-2017. [DOI: 10.1007/s10664-017-9574-5] - DOI
Mercieca‐Bebber 2018
Moncrieff 2003
Moncrieff 2004
Morris 2002
Moustgaard 2020
Page 2016
Salamone 2000
Schulz 2002
Sterne 2002
Sterne 2019
Storebø 2015
-
- Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD009885. [DOI: 10.1002/14651858.CD009885.pub2] [PMID: ] - DOI - PMC - PubMed
Thomson 1982a
Tytgat 2007
Wilkinson 2019
-
- Wilkinson ST, Farmer C, Ballard ED, Mathew SJ, Grunebaum MF, Murrough JW, et al. Impact of midazolam vs. saline on effect size estimates in controlled trials of ketamine as a rapid-acting antidepressant. Neuropsychopharmacology 2019;44(7):1233-8. [DOI: 10.1038/s41386-019-0317-8] [PMID: ] - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

