Electrochemical sensing platform with gold nanoparticles capped by PDDA for benzyl alcohol determination
- PMID: 36877248
- PMCID: PMC9988818
- DOI: 10.1007/s00604-023-05690-6
Electrochemical sensing platform with gold nanoparticles capped by PDDA for benzyl alcohol determination
Abstract
An electrochemical sensor has been developed, by modifying screen-printed carbon devices (SPCE) with photochemically synthesized gold nanoparticles (AuNP), to determine benzyl alcohol, a preservative widely used in the cosmetic industry. To obtain the AuNP with the best properties for electrochemical sensing applications, the photochemical synthesis was optimized using chemometric tools. A response surface methodology based on central composite design was used to optimize the synthesis conditions, as irradiation time, and the concentrations of metal precursor and the capping/reducing agent (poly(diallyldimethylammonium) chloride, PDDA). The anodic current of benzyl alcohol on SPCE modified with the AuNP was used as response of the system. The best electrochemical responses were obtained using the AuNP generated by irradiating for 18 min a 7.20 [Formula: see text] 10-4 mol L-1 AuCl4--1.7% PDDA solution. The AuNP were characterized by transmission electron microscopy, cyclic voltammetry and dynamic light scattering. The nanocomposite-based sensor formed by the optimal AuNP (AuNP@PDDA/SPCE) was used to determine benzyl alcohol by linear sweep voltammetry in 0.10 mol L-1 KOH. The anodic current at + 0.017 ± 0.003 V (vs. AgCl) was used as analytical signal. Detection limit obtained under these conditions was 2.8 µg mL-1. The AuNP@PDDA/SPCE was applied to determine benzyl alcohol in cosmetic samples.
Keywords: Benzyl alcohol; Central composite design; Electrochemical sensor; Gold nanoparticles; Linear sweep voltammetry; Response surface methodology.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

 non irradiated and at room temperature;
non irradiated and at room temperature;
 heated at 86 °C in the darkness; after irradiation for 1 min at
heated at 86 °C in the darkness; after irradiation for 1 min at
 86 °C and
86 °C and
 32 °C. B Absorption spectra of a 5.00 10−4 mol L−1 AuCl4− aqueous solution
32 °C. B Absorption spectra of a 5.00 10−4 mol L−1 AuCl4− aqueous solution
 before and
before and
 after 15 min of irradiation and spectra of a 5.00 10−4 mol L−1 AuCl4− 0.75% PDDA solution
after 15 min of irradiation and spectra of a 5.00 10−4 mol L−1 AuCl4− 0.75% PDDA solution
 before and
before and
 after 15 min of irradiation
after 15 min of irradiation
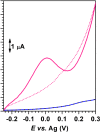
 (overlapped signals) and AuNP@PDDA/SPCE
(overlapped signals) and AuNP@PDDA/SPCE
 . Electrochemical response of AuNP@PDDA/SPCE in 0.10 mol L−1 KOH
. Electrochemical response of AuNP@PDDA/SPCE in 0.10 mol L−1 KOH
 . Scan rate: 0.100 V s−1
. Scan rate: 0.100 V s−1
References
-
- Annadhasan M, Kasthurib J, Rajendiran N. Green synthesis of gold nanoparticles under sunlight irradiation and their colorimetric detection of Ni2+ and Co2+ ions. RSC Adv. 2015;5:11458–11468. doi: 10.1039/C4RA14034F. - DOI
LinkOut - more resources
Full Text Sources
