HIV post-treatment controllers have distinct immunological and virological features
- PMID: 36877848
- PMCID: PMC10089217
- DOI: 10.1073/pnas.2218960120
HIV post-treatment controllers have distinct immunological and virological features
Abstract
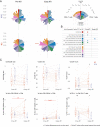
HIV post-treatment controllers (PTCs) are rare individuals who maintain low levels of viremia after stopping antiretroviral therapy (ART). Understanding the mechanisms of HIV post-treatment control will inform development of strategies aiming at achieving HIV functional cure. In this study, we evaluated 22 PTCs from 8 AIDS Clinical Trials Group (ACTG) analytical treatment interruption (ATI) studies who maintained viral loads ≤400 copies/mL for ≥24 wk. There were no significant differences in demographics or frequency of protective and susceptible human leukocyte antigen (HLA) alleles between PTCs and post-treatment noncontrollers (NCs, n = 37). Unlike NCs, PTCs demonstrated a stable HIV reservoir measured by cell-associated RNA (CA-RNA) and intact proviral DNA assay (IPDA) during analytical treatment interruption (ATI). Immunologically, PTCs demonstrated significantly lower CD4+ and CD8+ T cell activation, lower CD4+ T cell exhaustion, and more robust Gag-specific CD4+ T cell responses and natural killer (NK) cell responses. Sparse partial least squares discriminant analysis (sPLS-DA) identified a set of features enriched in PTCs, including a higher CD4+ T cell% and CD4+/CD8+ ratio, more functional NK cells, and a lower CD4+ T cell exhaustion level. These results provide insights into the key viral reservoir features and immunological profiles for HIV PTCs and have implications for future studies evaluating interventions to achieve an HIV functional cure.
Keywords: HIV; T cell; analytical treatment interruption; post-treatment controller; reservoir.
Conflict of interest statement
J.Z.L has received research support from Merck. M.M.L. and X.G.Y have received research support from Gilead Sciences. I.F. has received honoraria as a consultant to Gilead Sciences, ViiV Healthcare, and Merck and has received research support from Janssen Therapeutics, Sanofi, Moderna, and Pfizer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Figures






References
-
- Hutter G., et al. , Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 360, 692–698 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069501/AI/NIAID NIH HHS/United States
- U01 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- UM1 AI164560/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- T32 AI007387/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- R01 AI150396/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- P01 AI169768/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

