Biolistic delivery of liposomes protected in metal-organic frameworks
- PMID: 36877851
- PMCID: PMC10089211
- DOI: 10.1073/pnas.2218247120
Biolistic delivery of liposomes protected in metal-organic frameworks
Abstract
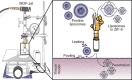
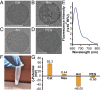
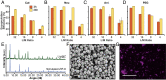
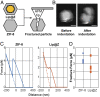
Needle-and-syringe-based delivery has been the commercial standard for vaccine administration to date. With worsening medical personnel availability, increasing biohazard waste production, and the possibility of cross-contamination, we explore the possibility of biolistic delivery as an alternate skin-based delivery route. Delicate formulations like liposomes are inherently unsuitable for this delivery model as they are fragile biomaterials incapable of withstanding shear stress and are exceedingly difficult to formulate as a lyophilized powder for room temperature storage. Here we have developed a approach to deliver liposomes into the skin biolistically-by encapsulating them in a nano-sized shell made of Zeolitic Imidazolate Framework-8 (ZIF-8). When encapsulated within a crystalline and rigid coating, the liposomes are not only protected from thermal stress, but also shear stress. This protection from stressors is crucial, especially for formulations with cargo encapsulated inside the lumen of the liposomes. Moreover, the coating provides the liposomes with a solid exterior that allows the particles to penetrate the skin effectively. In this work, we explored the mechanical protection ZIF-8 provides to liposomes as a preliminary investigation for using biolistic delivery as an alternative to syringe-and-needle-based delivery of vaccines. We demonstrated that liposomes with a variety of surface charges could be coated with ZIF-8 using the right conditions, and this coating can be just as easily removed-without causing any damage to the protected material. The protective coating prevented the liposomes from leaking cargo and helped in their effective penetration when delivered into the agarose tissue model and porcine skin tissue.
Keywords: biolistic delivery; biomimetic mineralization; metal-organic frameworks; shear stress; zeolitic imidazolate frameworks.
Conflict of interest statement
The authors declare no competing interest.
Figures
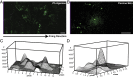
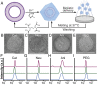
References
-
- Tenchov R., Bird R., Curtze A. E., Zhou Q., Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 15, 16982–17015 (2021). - PubMed
-
- Chatzikleanthous D., O’Hagan D. T., Adamo R., Lipid-based nanoparticles for delivery of vaccine adjuvants and antigens: Toward multicomponent vaccines. Mol. Pharm. 18, 2867–2888 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

