How I treat unique and difficult-to-manage cases of CAR T-cell therapy-associated neurotoxicity
- PMID: 36877916
- PMCID: PMC10329188
- DOI: 10.1182/blood.2022017604
How I treat unique and difficult-to-manage cases of CAR T-cell therapy-associated neurotoxicity
Abstract
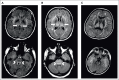
With growing indications for chimeric antigen receptor (CAR) T-cell therapy, toxicity profiles are evolving. There is an urgent and unmet need of approaches to optimally manage emerging adverse events that extend beyond the standard paradigm of cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome (ICANS). Although management guidelines exist for ICANS, there is little guidance on how to approach patients with neurologic comorbidities, and how to manage rare neurotoxicity presentations, such as CAR T-cell therapy-related cerebral edema, severe motor complications or late-onset neurotoxicity. In this study, we present 3 scenarios of patients treated with CAR T cells who develop unique types of neurotoxicity, and we describe an approach for the evaluation and management based on experience because objective data are limited. The goal of this study is to develop an awareness of emerging and unusual complications, discuss treatment approaches, and help institutions and health care providers establish frameworks to navigate how to best address unusual neurotoxicities to ultimately improve patient outcomes.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: F.P. received research funding from LRF, Lonza, and NGMBiopharmaceutical. B.D.S. provided consultancy/advisory for Celgene, BMS, Janssen, Legend, Pfizer, and In8bio. J.G. provided consultancy for Johnson & Johnson.
Figures

Comment in
-
Introduction to a How I Treat series on emergent CAR T-cell toxicities.Blood. 2023 May 18;141(20):2405-2407. doi: 10.1182/blood.2023020228. Blood. 2023. PMID: 36928098 No abstract available.
References
-
- Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–2221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

