Neural control of female sexual behaviors
- PMID: 36878049
- PMCID: PMC10133197
- DOI: 10.1016/j.yhbeh.2023.105339
Neural control of female sexual behaviors
Abstract
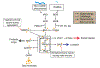
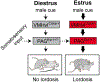
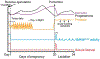
Reproduction is the biological process by which new individuals are produced by their parents. It is the fundamental feature of all known life and is required for the existence of all species. All mammals reproduce sexually, a process that involves the union of two reproductive cells, one from a male and one from a female. Sexual behaviors are a series of actions leading to reproduction. They are composed of appetitive, action, and refractory phases, each supported by dedicated developmentally-wired neural circuits to ensure high reproduction success. In rodents, successful reproduction can only occur during female ovulation. Thus, female sexual behavior is tightly coupled with ovarian activity, namely the estrous cycle. This is achieved through the close interaction between the female sexual behavior circuit and the hypothalamic-pituitary-gonadal (HPG) axis. In this review, we will summarize our current understanding, learned mainly in rodents, regarding the neural circuits underlying each phase of the female sexual behaviors and their interaction with the HPG axis, highlighting the gaps in our knowledge that require future investigation.
Keywords: Female sexual behaviors; HPG axis; Neural circuits; Rodents; Sex hormones.
Copyright © 2023 Elsevier Inc. All rights reserved.
Figures




References
-
- Bakker J, and Baum MJ (2000). Neuroendocrine regulation of GnRH release in induced ovulators. Front Neuroendocrinol 21, 220–262. - PubMed
-
- Bakker J, Pierman S, and González-Martínez D (2010). Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Hormones behavior 57, 390–395. - PubMed
-
- Baum MJ, and Keverne EB (2002). Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav 41, 213–219. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

