Ex vivo expansion of circulating tumour cells (CTCs)
- PMID: 36879003
- PMCID: PMC9988863
- DOI: 10.1038/s41598-023-30733-6
Ex vivo expansion of circulating tumour cells (CTCs)
Abstract
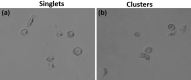
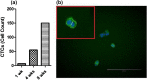
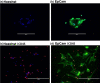
Circulating tumour cells (CTCs) are a critical intermediate step in the process of cancer metastasis. The reliability of CTC isolation/purification has limited both the potential to report on metastatic progression and the development of CTCs as targets for therapeutic intervention. Here we report a new methodology, which optimises the culture conditions for CTCs using primary cancer cells as a model system. We exploited the known biology that CTCs thrive in hypoxic conditions, with their survival and proliferation being reliant on the activation of hypoxia-inducible factor 1 alpha (HIF-1α). We isolated epithelial-like and quasi-mesenchymal CTC phenotypes from the blood of a cancer patient and successfully cultured these cells for more than 8 weeks. The presence of CTC clusters was required to establish and maintain long-term cultures. This novel methodology for the long-term culture of CTCs will aid in the development of downstream applications, including CTC theranostics.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. - PubMed
-
- Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat. Rev. Cancer. 2014;14:623–631. - PubMed
-
- Aceto N, Toner M, Maheswaran S, Haber DA. En route to metastasis: Circulating tumor cell clusters and epithelial-to-mesenchymal transition. Trends Cancer. 2015;1:44–52. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

