Hematopoietic cell transplantation and cellular therapies in Europe 2021. The second year of the SARS-CoV-2 pandemic. A Report from the EBMT Activity Survey
- PMID: 36879108
- PMCID: PMC9987384
- DOI: 10.1038/s41409-023-01943-3
Hematopoietic cell transplantation and cellular therapies in Europe 2021. The second year of the SARS-CoV-2 pandemic. A Report from the EBMT Activity Survey
Abstract
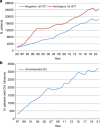
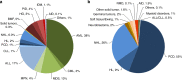
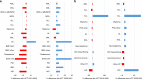
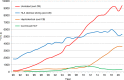
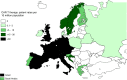
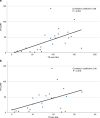
In 2021, 47,412 HCT (19,806 (42%) allogeneic and 27,606 (58%) autologous) in 43,109 patients were reported by 694 European centers. 3494 patients received advanced cellular therapies, 2524 of which were CAR-T treatments, an additional 3245 received DLI. Changes compared to the previous year were CAR-T treatment (+35%), allogeneic HCT +5.4%, autologous HCT +3.9%, more pronounced in non-malignant disorders. Main indications for allogeneic HCT were myeloid malignancies 10,745 (58%), lymphoid malignancies 5127 (28%) and non-malignant disorders 2501 (13%). Main indications for autologous HCT were lymphoid malignancies 22,129 (90%) and solid tumors 1635 (7%). In allogeneic HCT, use of haploidentical donors decreased by -0.9% while use of unrelated and sibling donors increased by +4.3% and +9%. Cord blood HCT decreased by -5.8%. Pediatric HCT increased overall by +5.6% (+6.9% allogeneic and +1.6% autologous). Increase in the use of CAR-T was mainly restricted to high-income countries. The drop in HCT activity reported in 2020 partially recovered in 2021, the second year of the SARS-CoV-2 pandemic. The transplant community confronted with the pandemic challenge, continued in providing patients access to treatment. This annual EBMT report reflects current activities useful for health care resource planning.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Gratwohl A. Bone marrow transplantation activity in Europe 1990. Report from the European Group for Bone Marrow Transplantation (EBMT) Bone Marrow Transplant. 1991;8:197–201. - PubMed
-
- Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022;57:1217–39. doi: 10.1038/s41409-022-01691-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

