Evinacumab in severe hypertriglyceridemia with or without lipoprotein lipase pathway mutations: a phase 2 randomized trial
- PMID: 36879129
- PMCID: PMC10033404
- DOI: 10.1038/s41591-023-02222-w
Evinacumab in severe hypertriglyceridemia with or without lipoprotein lipase pathway mutations: a phase 2 randomized trial
Abstract
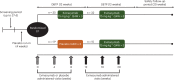
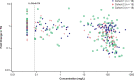
Severe hypertriglyceridemia (sHTG) is an established risk factor for acute pancreatitis. Current therapeutic approaches for sHTG are often insufficient to reduce triglycerides and prevent acute pancreatitis. This phase 2 trial ( NCT03452228 ) evaluated evinacumab (angiopoietin-like 3 inhibitor) in three cohorts of patients with sHTG: cohort 1, familial chylomicronemia syndrome with bi-allelic loss-of-function lipoprotein lipase (LPL) pathway mutations (n = 17); cohort 2, multifactorial chylomicronemia syndrome with heterozygous loss-of-function LPL pathway mutations (n = 15); and cohort 3, multifactorial chylomicronemia syndrome without LPL pathway mutations (n = 19). Fifty-one patients (males, n = 27; females, n = 24) with a history of hospitalization for acute pancreatitis were randomized 2:1 to intravenous evinacumab 15 mg kg-1 or placebo every 4 weeks over a 12-week double-blind treatment period, followed by a 12-week single-blind treatment period. The primary end point was the mean percent reduction in triglycerides from baseline after 12 weeks of evinacumab exposure in cohort 3. Evinacumab reduced triglycerides in cohort 3 by a mean (s.e.m.) of -27.1% (37.4) (95% confidence interval -71.2 to 84.6), but the prespecified primary end point was not met. No notable differences in adverse events between evinacumab and placebo treatment groups were seen during the double-blind treatment period. Although the primary end point of a reduction in triglycerides did not meet the prespecified significance level, the observed safety and changes in lipid and lipoprotein levels support the further evaluation of evinacumab in larger trials of patients with sHTG. Trial registration number: ClinicalTrials.gov NCT03452228 .
© 2023. The Author(s).
Conflict of interest statement
R.S.R. reports grants and personal fees from Regeneron Pharmaceuticals, grants and personal fees from Amgen, grants and personal fees from Arrowhead, grants and personal fees from Lilly, grants and personal fees from Novartis, personal fees from CRISPER Therapeutics, Kowa, Precision Biosciences, royalties from UpToDate, other from MediMergent, outside the submitted work. C.M.B. reports grants and personal fees from Abbott Diagnostic, grants from Akcea, grants and personal fees from Amarin, grants and personal fees from Amgen, grants and personal fees from Esperion, grants from Ionis, grants and personal fees from Novartis, grants and personal fees from Regeneron Pharmaceuticals, grants from Roche Diagnostic, grants and personal fees from Sanofi-Synthelabo, personal fees from Arrowhead, personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Denka Seiken, personal fees from Intercept, personal fees from Janssen, personal fees from Matinas BioPharma and personal fees from Merck, personal fees from Norvo Nordisk, outside the submitted work. P.B. is an employee/stockholder of Regeneron Pharmaceuticals. S.J.B. reports personal fees from Akcea, personal fees from Amgen, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, personal fees from Esperion, personal fees from Regeneron Pharmaceuticals, personal fees from Sanofi, personal fees from Novartis, personal fees from AstraZeneca, personal fees from Altimmune, personal fees from Madrigal Pharmaceuticals, personal fees from Axcella Health, other from Excel Medical Clinical Trials, outside the submitted work. J.B. reports grants and personal fees from Akcea-Ionis, grants and personal fees from Amgen, grants from HLS Therapeutic, grants from Kowa, grants and personal fees from Novartis, grants from Regeneron Pharmaceuticals and grants and personal fees from Sanofi, outside the submitted work. D.G. reports grants and personal fees from Regeneron Pharmaceuticals, during the conduct of the study; grants and personal fees from Akcea, grants from Acasti, grants and personal fees from Arrowhead, grants and personal fees from Ionis, grants from Kowa and grants from Uniqure, outside the submitted work. A.G. was an employee/stockholder of Regeneron Pharmaceuticals. C.G.-J. was an employee/stockholder of Regeneron Pharmaceuticals. E.K. reports grants and personal fees from Regeneron Pharmaceuticals, outside the submitted work. J.M. is an employee/stockholder of Regeneron Pharmaceuticals. P.M. reports personal fees from Aegerion, personal fees from Esperion, grants and personal fees from Kaneka, grants and personal fees from Stage 2 Innovations, grants and personal fees from Amgen, grants and personal fees from Regeneron Pharmaceuticals, grants from Akcea, grants from the FH Foundation, grants from Ionis, grants from Kowa and grants from Novartis, outside the submitted work. M.P. was an employee/stockholder of Regeneron Pharmaceuticals. R.P. reports other support from Regeneron Pharmaceuticals outside the submitted work. In addition, R.P. has a patent pending for US2020/0061189 ‘Methods for treating patients with familial hypercholesterolemia’. D.R. reports personal fees from Alnylam, personal fees from Novartis, personal fees from Pfizer, personal fees from Verve, grants and personal fees from Regeneron Pharmaceuticals, other funding from Staten Biotechnology and other funding from VascularStrategies, outside the submitted work. P.R. reports grants from Regeneron Pharmaceuticals during the conduct of the study and grants from Regeneron Pharmaceuticals, outside the submitted work. D.W. reports personal fees from AbbVie, personal fees from Abbott, personal fees from BioNTech, personal fees from Regeneron Pharmaceuticals, personal fees from Samsung and other funding from Ariel Precison Medicine, outside the submitted work. J.Z. is a contractor working with Regeneron Pharmaceuticals.
Figures


References
-
- US Food and Drug Administration. VASCEPA (icosapent ethyl) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202057s035lbl.pdf (2019).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

