Ubiquitin-specific protease 28: the decipherment of its dual roles in cancer development
- PMID: 36879346
- PMCID: PMC9990303
- DOI: 10.1186/s40164-023-00389-z
Ubiquitin-specific protease 28: the decipherment of its dual roles in cancer development
Abstract
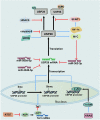
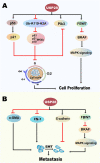
As significant posttranslational modifications, ubiquitination and deubiquitination, whose balance is modulated by ubiquitin-conjugating enzymes and deubiquitinating enzymes (DUBs), can regulate many biological processes, such as controlling cell cycle progression, signal transduction and transcriptional regulation. Belonging to DUBs, ubiquitin-specific protease 28 (USP28) plays an essential role in turning over ubiquitination and then contributing to the stabilization of quantities of substrates, including several cancer-related proteins. In previous studies, USP28 has been demonstrated to participate in the progression of various cancers. Nevertheless, several reports have recently shown that in addition to promoting cancers, USP28 can also play an oncostatic role in some cancers. In this review, we summarize the correlation between USP28 and tumor behaviors. We initially give a brief introduction of the structure and related biological functions of USP28, and we then introduce some concrete substrates of USP28 and the underlying molecular mechanisms. In addition, the regulation of the actions and expression of USP28 is also discussed. Moreover, we concentrate on the impacts of USP28 on diverse hallmarks of cancer and discuss whether USP28 can accelerate or inhibit tumor progression. Furthermore, clinical relevance, including impacting clinical prognosis, influencing therapy resistance and being the therapy target in some cancers, is depicted systematically. Thus, assistance may be given to future experimental designs by the information provided here, and the potential of targeting USP28 for cancer therapy is emphasized.
Keywords: Biomarker; Cancer; Prognosis; Therapy resistance; USP28.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Structure and function of the highly homologous deubiquitinases ubiquitin specific peptidase 25 and 28: Insights into their pathophysiological and therapeutic roles.Biochem Pharmacol. 2023 Jul;213:115624. doi: 10.1016/j.bcp.2023.115624. Epub 2023 May 26. Biochem Pharmacol. 2023. PMID: 37245535 Review.
-
Comprehensive prognostic and immunological analysis of Ubiquitin Specific Peptidase 28 in pan-cancers and identification of its role in hepatocellular carcinoma cell lines.Aging (Albany NY). 2023 Jul 13;15(13):6545-6576. doi: 10.18632/aging.204869. Epub 2023 Jul 13. Aging (Albany NY). 2023. PMID: 37450415 Free PMC article.
-
Targeting deubiquitinase USP28 for cancer therapy.Cell Death Dis. 2018 Feb 7;9(2):186. doi: 10.1038/s41419-017-0208-z. Cell Death Dis. 2018. PMID: 29415985 Free PMC article. Review.
-
Discovery of [1,2,3]triazolo[4,5-d]pyrimidine derivatives as highly potent, selective, and cellularly active USP28 inhibitors.Acta Pharm Sin B. 2020 Aug;10(8):1476-1491. doi: 10.1016/j.apsb.2019.12.008. Epub 2019 Dec 16. Acta Pharm Sin B. 2020. PMID: 32963944 Free PMC article.
-
Emerging Roles of Ubiquitin-Specific Protease 25 in Diseases.Front Cell Dev Biol. 2021 Jun 23;9:698751. doi: 10.3389/fcell.2021.698751. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249948 Free PMC article. Review.
Cited by
-
Deubiquitinases in cancer.Nat Rev Cancer. 2023 Dec;23(12):842-862. doi: 10.1038/s41568-023-00633-y. Epub 2023 Nov 7. Nat Rev Cancer. 2023. PMID: 37935888 Review.
-
Crosstalk between O-GlcNAcylation and ubiquitination: a novel strategy for overcoming cancer therapeutic resistance.Exp Hematol Oncol. 2024 Nov 1;13(1):107. doi: 10.1186/s40164-024-00569-5. Exp Hematol Oncol. 2024. PMID: 39487556 Free PMC article. Review.
-
Comprehensive analysis of the role of ubiquitin-specific peptidases in colorectal cancer: A systematic review.World J Gastrointest Oncol. 2024 Jan 15;16(1):197-213. doi: 10.4251/wjgo.v16.i1.197. World J Gastrointest Oncol. 2024. PMID: 38292842 Free PMC article.
-
Resveratrol inhibits Lin28A expression and induces its degradation via the proteasomal pathway in NCCIT cells.Oncol Lett. 2024 Sep 30;28(6):577. doi: 10.3892/ol.2024.14710. eCollection 2024 Dec. Oncol Lett. 2024. PMID: 39397804 Free PMC article.
-
USP28 protects development of inflammation in mouse intestine by regulating STAT5 phosphorylation and IL22 production in T lymphocytes.Front Immunol. 2024 Jul 15;15:1401949. doi: 10.3389/fimmu.2024.1401949. eCollection 2024. Front Immunol. 2024. PMID: 39076972 Free PMC article.
References
Publication types
Grants and funding
- 81871866, 82173252/National Natural Science Foundation of China
- 82103508/National Natural Science Foundation of China
- No. 2019 Special Support Plan/Shaanxi Special Support Plan-Program for Leading Talents of Science and Technology Innovation
- 2016SF-308/Natural Science Foundation of Shaanxi Province
- 2019SF-033/Natural Science Foundation of Shaanxi Province
LinkOut - more resources
Full Text Sources
Research Materials

