Treatment outcomes for newly diagnosed, treatment-naïve TP53-mutated acute myeloid leukemia: a systematic review and meta-analysis
- PMID: 36879351
- PMCID: PMC9990239
- DOI: 10.1186/s13045-023-01417-5
Treatment outcomes for newly diagnosed, treatment-naïve TP53-mutated acute myeloid leukemia: a systematic review and meta-analysis
Abstract
Background: TP53 mutations, which are present in 5% to 10% of patients with acute myeloid leukemia (AML), are associated with treatment resistance and poor outcomes. First-line therapies for TP53-mutated (TP53m) AML consist of intensive chemotherapy (IC), hypomethylating agents (HMA), or venetoclax combined with HMA (VEN + HMA).
Methods: We conducted a systematic review and meta-analysis to describe and compare treatment outcomes in newly diagnosed treatment-naïve patients with TP53m AML. Randomized controlled trials, single-arm trials, prospective observational studies, and retrospective studies were included that reported on complete remission (CR), CR with incomplete hematologic recovery (CRi), overall survival (OS), event-free survival (EFS), duration of response (DoR), and overall response rate (ORR) among patients with TP53m AML receiving first-line treatment with IC, HMA, or VEN + HMA.
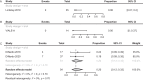
Results: Searches of EMBASE and MEDLINE identified 3006 abstracts, and 17 publications describing 12 studies met the inclusion criteria. Random-effects models were used to pool response rates, and time-related outcomes were analyzed with the median of medians method. IC was associated with the greatest CR rate of 43%, and CR rates were 33% for VEN + HMA and 13% for HMA. Rates of CR/CRi were comparable for IC (46%) and VEN + HMA (49%) but were lower for HMA (13%). Median OS was uniformly poor across treatments: IC, 6.5 months; VEN + HMA, 6.2 months; and HMA, 6.1 months. For IC, the EFS estimate was 3.7 months; EFS was not reported for VEN + HMA or HMA. The ORR was 41% for IC, 65% for VEN + HMA, and 47% for HMA. DoR was 3.5 months for IC, 5.0 months for VEN + HMA, and was not reported for HMA.
Conclusions: Despite improved responses seen with IC and VEN + HMA compared to HMA, survival was uniformly poor, and clinical benefits were limited across all treatments for patients with newly diagnosed, treatment-naïve TP53m AML, demonstrating a significant need for improved treatment for this difficult-to-treat population.
Keywords: Acute myeloid leukemia; Hypomethylating agents; Intensive chemotherapy; TP53 mutations; Venetoclax.
© 2023. The Author(s).
Conflict of interest statement
SI, RC, CR, KH, and GR are employees and stockholders of Gilead Sciences, Inc. CR is an Alphabet stockholder. NGD has received grants or contracts from Hanmi, Trovagene, Fate Therapeutics, Novimmune, and GlycoMimetics; consulting fees from Arog, Novartis, Jazz, Celgene, Syndax, Shattuck Labs, and Agios; and grants or contracts and consulting fees from Daiichi-Sankyo, Bristol-Meyers Squibb, Pfizer, Gilead Sciences, Inc., Servier, Genentech, Astellas, AbbVie, ImmunoGen, Amgen, and Trillium. HH, JY, PT, MJZ, and FX have nothing to disclose.
Figures



References
-
- National Cancer Institute. Acute myeloid leukemia treatment (PDQ) - health professional version. Updated January 18, 2022. https://www.cancer.gov/types/leukemia/hp/adult-aml-treatment-pdq - PubMed
-
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program (SEER). Cancer stat facts: leukemia - acute myeloid leukemia (AML). Updated 2022. https://seer.cancer.gov/statfacts/html/amyl.html
-
- Venclexta. Prescribing Information. AbbVie Inc. 2021. Accessed Feb 21, 2022. https://www.rxabbvie.com/pdf/venclexta.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

