Clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling of pancreatic cancer
- PMID: 36879539
- PMCID: PMC10073855
- DOI: 10.5946/ce.2022.086
Clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling of pancreatic cancer
Abstract
Background/aims: Endoscopic ultrasound-guided tissue acquisition (EUS-TA) is essential for the diagnosis of pancreatic cancer. The feasibility of comprehensive genomic profiling (CGP) using samples obtained by EUS-TA has been under recent discussion. This study aimed to evaluate the utility of EUS-TA for CGP in a clinical setting.
Methods: CGP was attempted in 178 samples obtained from 151 consecutive patients with pancreatic cancer at the Aichi Cancer Center between October 2019 and September 2021. We evaluated the adequacy of the samples for CGP and determined the factors associated with the adequacy of the samples obtained by EUS-TA retrospectively.
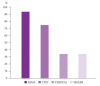
Results: The overall adequacy for CGP was 65.2% (116/178), which was significantly different among the four sampling methods (EUS-TA vs. surgical specimen vs. percutaneous biopsy vs. duodenal biopsy, 56.0% [61/109] vs. 80.4% [41/51] vs. 76.5% [13/17] vs. 100.0% [1/1], respectively; p=0.022). In a univariate analysis, needle gauge/type was associated with adequacy (22 G fine-needle aspiration vs. 22 G fine-needle biopsy [FNB] vs. 19 G-FNB, 33.3% (5/15) vs. 53.5% (23/43) vs. 72.5% (29/40); p=0.022). The sample adequacy of 19 G-FNB for CGP was 72.5% (29/40), and there was no significant difference between 19 G-FNB and surgical specimens (p=0.375).
Conclusion: To obtain adequate samples for CGP with EUS-TA, 19 G-FNB was shown to be the best in clinical practice. However, 19 G-FNB was not still sufficient, so further efforts are required to improve adequacy for CGP.
Keywords: Endoscopic ultrasound-guided fine needle aspiration; Genome; Pancreatic neoplasms.
Conflict of interest statement
Dr. Mizuno reports the following grants, none of which are connected to the submitted work: from Taiho Pharmaceutical, during the conduct of the study; grants and personal fees from Novartis; personal fees from AstraZeneca; grants from NanoCarrier; grants and personal fees from MSD; grants from Dainippon Sumitomo Pharma; grants from ASLAN Pharmaceuticals; grants from Incyte; grants from Ono Pharmaceutical; personal fees from Teijin Pharma; grants and personal fees from Yakult Honsha; personal fees from Fujifilm Toyama Chemical; and grants from Seagen.
Figures

Comment in
-
Endoscopic ultrasound-guided tissue acquisition for personalized treatment in pancreatic adenocarcinoma.Clin Endosc. 2023 Mar;56(2):183-184. doi: 10.5946/ce.2023.037. Epub 2023 Mar 15. Clin Endosc. 2023. PMID: 36941791 Free PMC article. No abstract available.
References
-
- Park DH, Lee TH, Paik WH, et al. Feasibility and safety of a novel dedicated device for one-step EUS-guided biliary drainage: a randomized trial. J Gastroenterol Hepatol. 2015;30:1461–1466. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
-
- Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973–981. - PubMed
LinkOut - more resources
Full Text Sources

