Dual-sized hollow particle incorporated fibroin thermal insulating coatings on catheter for cerebral therapeutic hypothermia
- PMID: 36879558
- PMCID: PMC9984786
- DOI: 10.1016/j.bioactmat.2023.02.022
Dual-sized hollow particle incorporated fibroin thermal insulating coatings on catheter for cerebral therapeutic hypothermia
Abstract
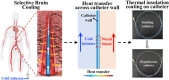
Selective endovascular hypothermia has been used to provide cooling-induced cerebral neuroprotection, but current catheters do not support thermally-insulated transfer of cold infusate, which results in an increased exit temperature, causes hemodilution, and limits its cooling efficiency. Herein, air-sprayed fibroin/silica-based coatings combined with chemical vapor deposited parylene-C capping film was prepared on catheter. This coating features in dual-sized-hollow-microparticle incorporated structures with low thermal conductivity. The infusate exit temperature is tunable by adjusting the coating thickness and infusion rate. No peeling or cracking was observed on the coatings under bending and rotational scenarios in the vascular models. Its efficiency was verified in a swine model, and the outlet temperature of coated catheter (75 μm thickness) was 1.8-2.0 °C lower than that of the uncoated one. This pioneering work on catheter thermal insulation coatings may facilitate the clinical translation of selective endovascular hypothermia for neuroprotection in patients with acute ischemic stroke.
Keywords: Catheter; Hypothermia; Silk fibroin; Stroke; Thermal insulation coating.
© 2023 The Authors.
Conflict of interest statement
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Design and evaluation of an air-insulated catheter for intra-arterial selective cooling infusion from numerical simulation and in vitro experiment.Med Eng Phys. 2022 Jan;99:103736. doi: 10.1016/j.medengphy.2021.103736. Epub 2021 Dec 6. Med Eng Phys. 2022. PMID: 35058029
-
Infusion warm during selective hypothermia in acute ischemic stroke.Brain Circ. 2019 Dec 27;5(4):218-224. doi: 10.4103/bc.bc_48_19. eCollection 2019 Oct-Dec. Brain Circ. 2019. PMID: 31950098 Free PMC article.
-
Catheter based selective hypothermia reduces stroke volume during focal cerebral ischemia in swine.J Neurointerv Surg. 2016 Apr;8(4):418-22. doi: 10.1136/neurintsurg-2014-011562. Epub 2015 Feb 12. J Neurointerv Surg. 2016. PMID: 25676148
-
Selective brain hypothermia.Handb Clin Neurol. 2018;157:839-852. doi: 10.1016/B978-0-444-64074-1.00052-5. Handb Clin Neurol. 2018. PMID: 30459044 Review.
-
Cold blood perfusion for selective hypothermia in acute ischemic stroke.Brain Circ. 2019 Dec 27;5(4):187-194. doi: 10.4103/bc.bc_17_19. eCollection 2019 Oct-Dec. Brain Circ. 2019. PMID: 31950094 Free PMC article. Review.
Cited by
-
Trends and hotspots of the neuroprotection of hypothermia treatment: A bibliometric and visualized analysis of research from 1992 to 2023.CNS Neurosci Ther. 2024 Jun;30(6):e14795. doi: 10.1111/cns.14795. CNS Neurosci Ther. 2024. PMID: 38867401 Free PMC article.
-
TiO2 Nanotube Implants Modified with Silk Fibroin and Mesoporous Silica Nanocomposite Coatings Enable Efficient Drug Release to Promote Osteogenesis.ACS Appl Mater Interfaces. 2025 May 28;17(21):30600-30612. doi: 10.1021/acsami.5c03599. Epub 2025 Apr 27. ACS Appl Mater Interfaces. 2025. PMID: 40289330 Free PMC article.
-
MgSO4 as a novel hypothermia infusion solution promotes ischemic stroke recovery through Ca2+ regulation of neurovascular units.Theranostics. 2025 Jan 2;15(5):1896-1913. doi: 10.7150/thno.104879. eCollection 2025. Theranostics. 2025. PMID: 39897562 Free PMC article.
References
-
- Saini V., Guada L., Yavagal D.R. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 Suppl 2):6–16. - PubMed
-
- Wu D., Li M., Fisher M., Ji X. Brain cytoprotection of ischemic stroke in the era of effective reperfusion. Sci. Bull. 2022;67(23):2372–2375. - PubMed
-
- Goyal M., Menon B.K., van Zwam W.H., Dippel D.W., Mitchell P.J., Demchuk A.M., Davalos A., Majoie C.B., van der Lugt A., de Miquel M.A., Donnan G.A., Roos Y.B., Bonafe A., Jahan R., Diener H.C., van den Berg L.A., Levy E.I., Berkhemer O.A., Pereira V.M., Rempel J., Millan M., Davis S.M., Roy D., Thornton J., Roman L.S., Ribo M., Beumer D., Stouch B., Brown S., Campbell B.C., van Oostenbrugge R.J., Saver J.L., Hill M.D., Jovin T.G. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet (London, England) 2016;387(10029):1723–1731. - PubMed
LinkOut - more resources
Full Text Sources

