Tunable hybrid hydrogels with multicellular spheroids for modeling desmoplastic pancreatic cancer
- PMID: 36879666
- PMCID: PMC9984297
- DOI: 10.1016/j.bioactmat.2023.02.005
Tunable hybrid hydrogels with multicellular spheroids for modeling desmoplastic pancreatic cancer
Abstract
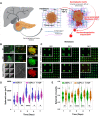
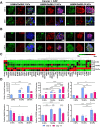
The tumor microenvironment consists of diverse, complex etiological factors. The matrix component of pancreatic ductal adenocarcinoma (PDAC) plays an important role not only in physical properties such as tissue rigidity but also in cancer progression and therapeutic responsiveness. Although significant efforts have been made to model desmoplastic PDAC, existing models could not fully recapitulate the etiology to mimic and understand the progression of PDAC. Here, two major components in desmoplastic pancreatic matrices, hyaluronic acid- and gelatin-based hydrogels, are engineered to provide matrices for tumor spheroids composed of PDAC and cancer-associated fibroblasts (CAF). Shape analysis profiles reveals that incorporating CAF contributes to a more compact tissue formation. Higher expression levels of markers associated with proliferation, epithelial to mesenchymal transition, mechanotransduction, and progression are observed for cancer-CAF spheroids cultured in hyper desmoplastic matrix-mimicking hydrogels, while the trend can be observed when those are cultured in desmoplastic matrix-mimicking hydrogels with the presence of transforming growth factor-β1 (TGF-β1). The proposed multicellular pancreatic tumor model, in combination with proper mechanical properties and TGF-β1 supplement, makes strides in developing advanced pancreatic models for resembling and monitoring the progression of pancreatic tumors, which could be potentially applicable for realizing personalized medicine and drug testing applications.
Keywords: Desmoplasia; Extracellular matrix; Fibrosis; Pancreatic cancer; Tumor microenvironment.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Viscoelastic stiffening of gelatin hydrogels for dynamic culture of pancreatic cancer spheroids.Acta Biomater. 2024 Mar 15;177:203-215. doi: 10.1016/j.actbio.2024.02.010. Epub 2024 Feb 12. Acta Biomater. 2024. PMID: 38354874 Free PMC article.
-
Bioengineered tumoral microtissues recapitulate desmoplastic reaction of pancreatic cancer.Acta Biomater. 2017 Feb;49:152-166. doi: 10.1016/j.actbio.2016.11.072. Epub 2016 Dec 1. Acta Biomater. 2017. PMID: 27916739
-
Stratified 3D Microtumors as Organotypic Testing Platforms for Screening Pancreatic Cancer Therapies.Small Methods. 2021 May;5(5):e2001207. doi: 10.1002/smtd.202001207. Epub 2021 Feb 10. Small Methods. 2021. PMID: 34928079
-
Apoptosis in the Pancreatic Cancer Tumor Microenvironment-The Double-Edged Sword of Cancer-Associated Fibroblasts.Cells. 2021 Jul 1;10(7):1653. doi: 10.3390/cells10071653. Cells. 2021. PMID: 34359823 Free PMC article. Review.
-
Heterogeneity and plasticity of cancer-associated fibroblasts in the pancreatic tumor microenvironment.Semin Cancer Biol. 2022 Jul;82:184-196. doi: 10.1016/j.semcancer.2021.03.006. Epub 2021 Mar 15. Semin Cancer Biol. 2022. PMID: 33737108 Review.
Cited by
-
Magnetic nanoparticles for ferroptosis cancer therapy with diagnostic imaging.Bioact Mater. 2023 Sep 29;32:66-97. doi: 10.1016/j.bioactmat.2023.09.015. eCollection 2024 Feb. Bioact Mater. 2023. PMID: 37822917 Free PMC article. Review.
-
Hydrogel models of pancreatic adenocarcinoma to study cell mechanosensing.Biophys Rev. 2024 Dec 18;16(6):851-870. doi: 10.1007/s12551-024-01265-8. eCollection 2024 Dec. Biophys Rev. 2024. PMID: 39830124 Free PMC article. Review.
-
Biomimetic Tumour Model Systems for Pancreatic Ductal Adenocarcinoma in Relation to Photodynamic Therapy.Int J Mol Sci. 2025 Jul 2;26(13):6388. doi: 10.3390/ijms26136388. Int J Mol Sci. 2025. PMID: 40650165 Free PMC article. Review.
-
Enhancing drug penetration in solid tumors via nanomedicine: Evaluation models, strategies and perspectives.Bioact Mater. 2023 Oct 26;32:445-472. doi: 10.1016/j.bioactmat.2023.10.017. eCollection 2024 Feb. Bioact Mater. 2023. PMID: 37965242 Free PMC article. Review.
-
Glucocorticoids in lung cancer: Navigating the balance between immunosuppression and therapeutic efficacy.Heliyon. 2024 Jun 4;10(12):e32357. doi: 10.1016/j.heliyon.2024.e32357. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39022002 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

