The mechanism underlying toxicity of a venom peptide against insects reveals how ants are master at disrupting membranes
- PMID: 36879819
- PMCID: PMC9985030
- DOI: 10.1016/j.isci.2023.106157
The mechanism underlying toxicity of a venom peptide against insects reveals how ants are master at disrupting membranes
Abstract
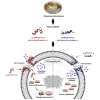
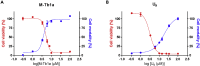
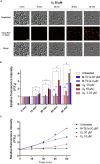
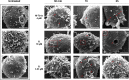
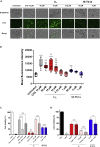
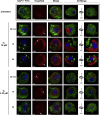
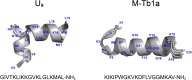
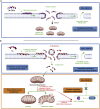
Hymenopterans represent one of the most abundant groups of venomous organisms but remain little explored due to the difficult access to their venom. The development of proteo-transcriptomic allowed us to explore diversity of their toxins offering interesting perspectives to identify new biological active peptides. This study focuses on U9 function, a linear, amphiphilic and polycationic peptide isolated from ant Tetramorium bicarinatum venom. It shares physicochemical properties with M-Tb1a, exhibiting cytotoxic effects through membrane permeabilization. In the present study, we conducted a comparative functional investigation of U9 and M-Tb1a and explored the mechanisms underlying their cytotoxicity against insect cells. After showing that both peptides induced the formation of pores in cell membrane, we demonstrated that U9 induced mitochondrial damage and, at high concentrations, localized into cells and induced caspase activation. This functional investigation highlighted an original mechanism of U9 questioning on potential valorization and endogen activity in T. bicarinatum venom.
Keywords: Biochemistry; Entomology; Toxicology.
© 2023.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Bordon K.d.C.F., Cologna C.T., Fornari-Baldo E.C., Pinheiro-Júnior E.L., Cerni F.A., Amorim F.G., Anjolette F.A.P., Cordeiro F.A., Wiezel G.A., Cardoso I.A., et al. From animal poisons and venoms to medicines: achievements, challenges and perspectives in drug discovery. Front. Pharmacol. 2020;11:1132. doi: 10.3389/fphar.2020.01132. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

