Specific reprogramming of alpha cells to insulin-producing cells by short glucagon promoter-driven Pdx1 and MafA
- PMID: 36879848
- PMCID: PMC9984919
- DOI: 10.1016/j.omtm.2023.02.003
Specific reprogramming of alpha cells to insulin-producing cells by short glucagon promoter-driven Pdx1 and MafA
Abstract
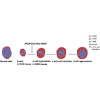
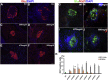
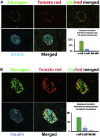
Endogenous reprogramming of pancreas-derived non-beta cells into insulin-producing cells is a promising approach to treat type 1 diabetes (T1D). One strategy that has yet to be explored is the specific delivery of insulin-producing essential genes, Pdx1 and MafA, to pancreatic alpha cells to reprogram the cells into insulin-producing cells in an adult pancreas. In this study, we used an alpha cell-specific glucagon (GCG) promoter to drive Pdx1 and MafA transcription factors to reprogram alpha cells to insulin-producing cells in chemically induced and autoimmune diabetic mice. Our results showed that a combination of a short glucagon-specific promoter with AAV serotype 8 (AAV8) can be used to successfully deliver Pdx1 and MafA to pancreatic alpha cells in the mouse pancreas. Pdx1 and MafA expression specifically in alpha cells were also able to correct hyperglycemia in both induced and autoimmune diabetic mice. With this technology, targeted gene specificity and reprogramming were accomplished with an alpha-specific promotor combined with an AAV-specific serotype and provide an initial basis to develop a novel therapy for the treatment of T1D.
Keywords: adeno-associated virus (AAV) vector; glucagon (GCG) promoter; pancreatic and duodenal homeobox 1 (Pdx1); protein a (MafA); type 1 diabetes (T1D); v-maf musculoaponeurotic fibrosarcoma oncogene family.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Gerrish K., Gannon M., Shih D., Henderson E., Stoffel M., Wright C.V., Stein R. Pancreatic beta cell-specific transcription of the pdx-1 gene. The role of conserved upstream control regions and their hepatic nuclear factor 3beta sites. J. Biol. Chem. 2000;275:3485–3492. doi: 10.1074/jbc.275.5.3485. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

