Long Non-Coding RNAs Within Macrophage-Derived Exosomes Promote BMSC Osteogenesis in a Bone Fracture Rat Model
- PMID: 36879890
- PMCID: PMC9985426
- DOI: 10.2147/IJN.S398446
Long Non-Coding RNAs Within Macrophage-Derived Exosomes Promote BMSC Osteogenesis in a Bone Fracture Rat Model
Abstract
Purpose: To investigate the effect of macrophage exosomal long non-coding (lnc)RNAs on bone mesenchymal stem cell (BMSC) osteogenesis and the associated mechanism.
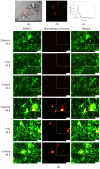
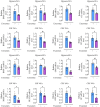
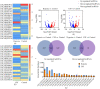
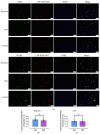
Methods: Rat BMSCs and spleen macrophages were co-cultured with serum derived from the fracture microenvironment of rat tibia. BMSC osteogenesis was evaluated using Alizarin red staining and the expression of BMP-2, RUNX2, OPN, and OC mRNA. BMSC osteogenesis was evaluated after co-culture with macrophages stimulated using hypoxic conditions or colony-stimulating factor (CSF). The uptake of macrophage-derived exosomes by BMSCs was evaluated using the exosome uptake assay. High-throughput sequencing and bioinformatics analyses were performed to identify key lncRNAs in the macrophage exosomes. The effect of lncRNA expression levels on BMSC osteogenesis was also assessed using a lncRNA overexpression plasmid and siRNA technology. M1 and M2 macrophages were distinguished using flow cytometry and the key exosomal lncRNA was detected by in situ hybridization.
Results: In the fracture microenvironment, macrophages (stimulated using either hypoxia or CSF) significantly increased the osteogenic ability of BMSCs. We showed that BMSCs assimilated macrophage-derived vesicles and that the inhibition of exosomal secretion significantly attenuated the macrophage-mediated induction of BMSC osteogenesis. The hypoxia condition led to the up-regulation of 310 lncRNAs and the down-regulation of 575 lncRNAs in macrophage exosomes, while CSF stimulation caused the up-regulation of 557 lncRNAs and the down-regulation of 407 lncRNAs. In total, 108 lncRNAs were co-up-regulated and 326 lncRNAs were co-down-regulated under both conditions. We eventually identified LOC103691165 as a key lncRNA that promoted BMSC osteogenesis and was expressed at similar levels in both M1 and M2 macrophages.
Conclusion: In the fracture microenvironment, M1 and M2 macrophages promoted BMSC osteogenesis by secreting exosomes containing LOC103691165.
Keywords: BMSCs osteogenesis; LOC103691165; bone fracture microenvironment; lncRNAs; macrophage exosomes.
© 2023 Wang et al.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

